Automated transmission-mode scanning electron microscopy (tSEM) for large volume analysis at nanoscale resolution
- PMID: 23555711
- PMCID: PMC3608656
- DOI: 10.1371/journal.pone.0059573
Automated transmission-mode scanning electron microscopy (tSEM) for large volume analysis at nanoscale resolution
Abstract
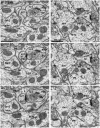
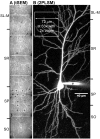
Transmission-mode scanning electron microscopy (tSEM) on a field emission SEM platform was developed for efficient and cost-effective imaging of circuit-scale volumes from brain at nanoscale resolution. Image area was maximized while optimizing the resolution and dynamic range necessary for discriminating key subcellular structures, such as small axonal, dendritic and glial processes, synapses, smooth endoplasmic reticulum, vesicles, microtubules, polyribosomes, and endosomes which are critical for neuronal function. Individual image fields from the tSEM system were up to 4,295 µm(2) (65.54 µm per side) at 2 nm pixel size, contrasting with image fields from a modern transmission electron microscope (TEM) system, which were only 66.59 µm(2) (8.160 µm per side) at the same pixel size. The tSEM produced outstanding images and had reduced distortion and drift relative to TEM. Automated stage and scan control in tSEM easily provided unattended serial section imaging and montaging. Lens and scan properties on both TEM and SEM platforms revealed no significant nonlinear distortions within a central field of ∼100 µm(2) and produced near-perfect image registration across serial sections using the computational elastic alignment tool in Fiji/TrakEM2 software, and reliable geometric measurements from RECONSTRUCT™ or Fiji/TrakEM2 software. Axial resolution limits the analysis of small structures contained within a section (∼45 nm). Since this new tSEM is non-destructive, objects within a section can be explored at finer axial resolution in TEM tomography with current methods. Future development of tSEM tomography promises thinner axial resolution producing nearly isotropic voxels and should provide within-section analyses of structures without changing platforms. Brain was the test system given our interest in synaptic connectivity and plasticity; however, the new tSEM system is readily applicable to other biological systems.
Conflict of interest statement
Figures




Similar articles
-
Large-volume reconstruction of brain tissue from high-resolution serial section images acquired by SEM-based scanning transmission electron microscopy.Methods Mol Biol. 2013;950:253-73. doi: 10.1007/978-1-62703-137-0_15. Methods Mol Biol. 2013. PMID: 23086880 Free PMC article.
-
A comparison of energy dispersive spectroscopy in transmission scanning electron microscopy with scanning transmission electron microscopy.Ultramicroscopy. 2025 Apr;270:114106. doi: 10.1016/j.ultramic.2025.114106. Epub 2025 Jan 17. Ultramicroscopy. 2025. PMID: 39862556
-
Restoring drifted electron microscope volumes using synaptic vesicles at sub-pixel accuracy.Commun Biol. 2020 Feb 21;3(1):81. doi: 10.1038/s42003-020-0809-4. Commun Biol. 2020. PMID: 32081999 Free PMC article.
-
Volume scanning electron microscopy for imaging biological ultrastructure.Biol Cell. 2016 Nov;108(11):307-323. doi: 10.1111/boc.201600024. Epub 2016 Aug 12. Biol Cell. 2016. PMID: 27432264 Review.
-
Challenges of microtome-based serial block-face scanning electron microscopy in neuroscience.J Microsc. 2015 Aug;259(2):137-142. doi: 10.1111/jmi.12244. Epub 2015 Apr 23. J Microsc. 2015. PMID: 25907464 Free PMC article. Review.
Cited by
-
Long-Term Potentiation Produces a Sustained Expansion of Synaptic Information Storage Capacity in Adult Rat Hippocampus.bioRxiv [Preprint]. 2024 Jan 14:2024.01.12.574766. doi: 10.1101/2024.01.12.574766. bioRxiv. 2024. PMID: 38260636 Free PMC article. Preprint.
-
Balancing Extrasynaptic Excitation and Synaptic Inhibition within Olfactory Bulb Glomeruli.eNeuro. 2019 Aug 7;6(4):ENEURO.0247-19.2019. doi: 10.1523/ENEURO.0247-19.2019. Print 2019 Jul/Aug. eNeuro. 2019. PMID: 31345999 Free PMC article.
-
Correlated light and electron microscopy: ultrastructure lights up!Nat Methods. 2015 Jun;12(6):503-13. doi: 10.1038/nmeth.3400. Nat Methods. 2015. PMID: 26020503 Review.
-
Three-dimensional synaptic analyses of mitral cell and external tufted cell dendrites in rat olfactory bulb glomeruli.J Comp Neurol. 2017 Feb 15;525(3):592-609. doi: 10.1002/cne.24089. Epub 2016 Aug 18. J Comp Neurol. 2017. PMID: 27490056 Free PMC article.
-
Genetically targeted 3D visualisation of Drosophila neurons under Electron Microscopy and X-Ray Microscopy using miniSOG.Sci Rep. 2016 Dec 13;6:38863. doi: 10.1038/srep38863. Sci Rep. 2016. PMID: 27958322 Free PMC article.
References
-
- Keddie FM, Barajas L (1969) Three-dimensional reconstruction of Pityrosporum yeast cells based on serial section electron microscopy. J Ultrastruct Res 29: 260–275. - PubMed
-
- Osafune T, Schwartzbach SD (2010) Serial section immunoelectron microscopy of algal cells. In: Schwartzbach SD, Osafune T, editors. Immunoelectron microscopy. Methods in molecular biology. Humana Press, Vol. 657: 259–274. - PubMed
-
- Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, et al. (2012) The connectome of a decision-making neural network. Science 337: 437–444. - PubMed
-
- Chiang RG, Govind CK (1986) Reorganization of synaptic ultrastructure at facilitated lobster neuromuscular terminals. J Neurocytol 15: 63–74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

