Human adipose tissue derived mesenchymal stem cells aggravate chronic cyclosporin nephrotoxicity by the induction of oxidative stress
- PMID: 23555748
- PMCID: PMC3608559
- DOI: 10.1371/journal.pone.0059693
Human adipose tissue derived mesenchymal stem cells aggravate chronic cyclosporin nephrotoxicity by the induction of oxidative stress
Abstract
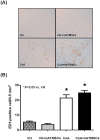
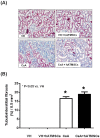
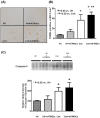
The aim of this study was to investigate whether hATMSCs protect against cyclosporine (CsA)-induced renal injury. CsA (7.5 mg/kg) and hATMSCs (3×10(6)/5 mL) were administered alone and together to rats for 4 weeks. The effect of hATMSCs on CsA-induced renal injury was evaluated by assessing renal function, interstitial fibrosis, infiltration of inflammatory cells, and apoptotic cell death. Four weeks of CsA-treatment produced typical chronic CsA-nephropathy. Combined treatment with CsA and hATMSCs did not prevent these effects and showed a trend toward further renal deterioration. To evaluate why hATMSCs aggravated CsA-induced renal injury, we measured oxidative stress, a major mechanism of CsA-induced renal injury. Both urine and serum 8-hydroxydeoxyguanosine(8-OHdG) levels were higher in the CsA+hATMSCs group than in the CsA group (P<0.05). An in vitro study showed similar results. Although the rate of apoptosis did not differ significantly between HK-2 cells cultured in hATMSCs-conditioned medium and those cultured in DMEM, addition of CsA resulted in greater apoptosis in HK-2 cells cultured in hATMSCs-conditioned medium. Addition of CsA increased oxidative stress in the hATMSCs-conditioned medium. The results of our study suggest that treatment with hATMSCs may aggravate CsA-induced renal injury because hATMSCs cause oxidative stress in the presence of CsA.
Conflict of interest statement
Figures




References
-
- Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM (1996) Chronic cyclosporine nephropathy: the Achilles’ heel of immunosuppressive therapy. Kidney Int 50: 1089–1100. - PubMed
-
- Myers BD, Ross J, Newton L, Luetscher J, Perlroth M (1984) Cyclosporine-associated chronic nephropathy. N Engl J Med 311: 699–705. - PubMed
-
- Wang C, Salahudeen AK (1995) Lipid peroxidation accompanies cyclosporine nephrotoxicity: effects of vitamin E. Kidney Int. 47: 927–934. - PubMed
-
- Yang CW, Faulkner GR, Wahba IM, Christianson TA, Bagby GC, et al. (2002) Expression of apoptosis-related genes in chronic cyclosporine nephrotoxicity in mice. Am J Transplant 2: 391–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

