Bapineuzumab alters aβ composition: implications for the amyloid cascade hypothesis and anti-amyloid immunotherapy
- PMID: 23555764
- PMCID: PMC3605408
- DOI: 10.1371/journal.pone.0059735
Bapineuzumab alters aβ composition: implications for the amyloid cascade hypothesis and anti-amyloid immunotherapy
Abstract
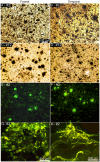
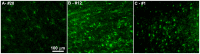
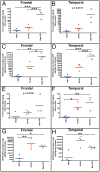
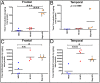
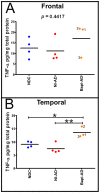
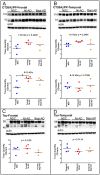
The characteristic neuropathological changes associated with Alzheimer's disease (AD) and other lines of evidence support the amyloid cascade hypothesis. Viewing amyloid deposits as the prime instigator of dementia has now led to clinical trials of multiple strategies to remove or prevent their formation. We performed neuropathological and biochemical assessments of 3 subjects treated with bapineuzumab infusions. Histological analyses were conducted to quantify amyloid plaque densities, Braak stages and the extent of cerebral amyloid angiopathy (CAA). Amyloid-β (Aβ) species in frontal and temporal lobe samples were quantified by ELISA. Western blots of amyloid-β precursor protein (AβPP) and its C-terminal (CT) fragments as well as tau species were performed. Bapineuzumab-treated (Bapi-AD) subjects were compared to non-immunized age-matched subjects with AD (NI-AD) and non-demented control (NDC) cases. Our study revealed that Bapi-AD subjects exhibited overall amyloid plaque densities similar to those of NI-AD cases. In addition, CAA was moderate to severe in NI-AD and Bapi-AD patients. Although histologically-demonstrable leptomeningeal, cerebrovascular and neuroparenchymal-amyloid densities all appeared unaffected by treatment, Aβ peptide profiles were significantly altered in Bapi-AD subjects. There was a trend for reduction in total Aβ42 levels as well as an increase in Aβ40 which led to a corresponding significant decrease in Aβ42:Aβ40 ratio in comparison to NI-AD subjects. There were no differences in the levels of AβPP, CT99 and CT83 or tau species between Bapi-AD and NI-AD subjects. The remarkable alteration in Aβ profiles reveals a dynamic amyloid production in which removal and depositional processes were apparently perturbed by bapineuzumab therapy. Despite the alteration in biochemical composition, all 3 immunized subjects exhibited continued cognitive decline.
Conflict of interest statement
Figures


References
-
- Pul R, Dodel R, Stangel M (2011) Antibody-based therapy in Alzheimer’s disease. Expert Opin Biol Ther 11: 343–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

