Vitiligo: a possible model of degenerative diseases
- PMID: 23555779
- PMCID: PMC3608562
- DOI: 10.1371/journal.pone.0059782
Vitiligo: a possible model of degenerative diseases
Abstract
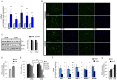
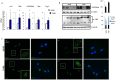
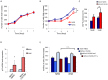
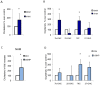
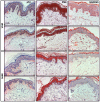
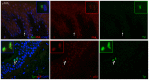
Vitiligo is characterized by the progressive disappearance of pigment cells from skin and hair follicle. Several in vitro and in vivo studies show evidence of an altered redox status, suggesting that loss of cellular redox equilibrium might be the pathogenic mechanism in vitiligo. However, despite the numerous data supporting a pathogenic role of oxidative stress, there is still no consensus explanation underlying the oxidative stress-driven disappear of melanocytes from the epidermis. In this study, in vitro characterization of melanocytes cultures from non-lesional vitiligo skin revealed at the cellular level aberrant function of signal transduction pathways common with neurodegenerative diseases including modification of lipid metabolism, hyperactivation of mitogen-activated protein kinase (MAPK) and cAMP response element-binding protein (CREB), constitutive p53-dependent stress signal transduction cascades, and enhanced sensibility to pro-apoptotic stimuli. Notably, these long-term effects of subcytotoxic oxidative stress are also biomarkers of pre-senescent cellular phenotype. Consistent with this, vitiligo cells showed a significant increase in p16 that did not correlate with the chronological age of the donor. Moreover, vitiligo melanocytes produced many biologically active proteins among the senescence-associated secretory phenotype (SAPS), such as interleukin-6 (IL-6), matrix metallo proteinase-3 (MMP3), cyclooxygenase-2 (Cox-2), insulin-like growth factor-binding protein-3 and 7 (IGFBP3, IGFBP7). Together, these data argue for a complicated pathophysiologic puzzle underlying melanocytes degeneration resembling, from the biological point of view, neurodegenerative diseases. Our results suggest new possible targets for intervention that in combination with current therapies could correct melanocytes intrinsic defects.
Conflict of interest statement
Figures



References
-
- Taieb A, Picardo M (2009) Clinical practice: Vitiligo. N Engl J Med 360: 160–169. - PubMed
-
- Le Poole IC, Luiten RM (2008) Autoimmune etiology of generalized vitiligo. Curr Dir Autoimmun 2008 10: 227–243. - PubMed
-
- Dell'Anna ML, Ottaviani M, Albanesi V, Vidolin AP, Leone G, et al. (2007) Membrane lipid alterations as a possible basis for melanocyte dagenaration in vitiligo. J Invest Dermatol 127: 1226–1233. - PubMed
-
- Schallreuter KU, Rübsam K, Gibbons NC, Maitland DJ, Chavan B, et al. (2008) Methionine sulfoxide reductases A and B deactivated by hydrogen peroxide (H2O2) in the epidermis of patients with vitiligo. J Invest Dermatol 128: 808–815. - PubMed
-
- Gauthier Y, Cario Andre M, Taϊeb A (2003) A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res 16: 322–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

