Specific in vivo labeling of tyrosinated α-tubulin and measurement of microtubule dynamics using a GFP tagged, cytoplasmically expressed recombinant antibody
- PMID: 23555790
- PMCID: PMC3610906
- DOI: 10.1371/journal.pone.0059812
Specific in vivo labeling of tyrosinated α-tubulin and measurement of microtubule dynamics using a GFP tagged, cytoplasmically expressed recombinant antibody
Abstract
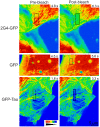
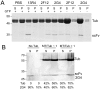
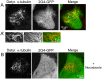
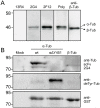
GFP-tagged proteins are used extensively as biosensors for protein localization and function, but the GFP moiety can interfere with protein properties. An alternative is to indirectly label proteins using intracellular recombinant antibodies (scFvs), but most antibody fragments are insoluble in the reducing environment of the cytosol. From a synthetic hyperstable human scFv library we isolated an anti-tubulin scFv, 2G4, which is soluble in mammalian cells when expressed as a GFP-fusion protein. Here we report the use of this GFP-tagged scFv to label microtubules in fixed and living cells. We found that 2G4-GFP localized uniformly along microtubules and did not disrupt binding of EB1, a protein that binds microtubule ends and serves as a platform for binding by a complex of proteins regulating MT polymerization. TOGp and CLIP-170 also bound microtubule ends in cells expressing 2G4-GFP. Microtubule dynamic instability, measured by tracking 2G4-GFP labeled microtubules, was nearly identical to that measured in cells expressing GFP-α-tubulin. Fluorescence recovery after photobleaching demonstrated that 2G4-GFP turns over rapidly on microtubules, similar to the turnover rates of fluorescently tagged microtubule-associated proteins. These data indicate that 2G4-GFP binds relatively weakly to microtubules, and this conclusion was confirmed in vitro. Purified 2G4 partially co-pelleted with microtubules, but a significant fraction remained in the soluble fraction, while a second anti-tubulin scFv, 2F12, was almost completely co-pelleted with microtubules. In cells, 2G4-GFP localized to most microtubules, but did not co-localize with those composed of detyrosinated α-tubulin, a post-translational modification associated with non-dynamic, more stable microtubules. Immunoblots probing bacterially expressed tubulins confirmed that 2G4 recognized α-tubulin and required tubulin's C-terminal tyrosine residue for binding. Thus, a recombinant antibody with weak affinity for its substrate can be used as a specific intracellular biosensor that can differentiate between unmodified and post-translationally modified forms of a protein.
Conflict of interest statement
Figures





References
-
- Nizak C, Monier S, del Nery E, Moutel S, Goud B, et al. (2003) Recombinant antibodies to the small GTPase Rab6 as conformation sensors. Science 300: 984–987. - PubMed
-
- Philibert P, Stoessel A, Wang W, Sibler A-P, Bec N, et al.. (2007) A focused antibody library for selecting scFvs expressed at high levels in the cytoplasm. BMC Biotechnology 7:81. Available: http://www.biomedcentral.com/1472-6750/7/81. Accessed 1 August 1 2012. - PMC - PubMed
-
- Dimitrov A, Quesnoit M, Moutel S, Cantaloube I, Pous C, et al. (2008) Detection of GTP-tubulin conformation in vivo reveals a role for GTP remnants in microtubule rescues. Science 322: 1353–1356. - PubMed
-
- Glockshuber R, Schmidt T, Pluckthun A (1992) The disulfide bonds in antibody variable domains: effects on stability, folding in vitro, and functional expression in Escherichia coli. Biochem 11: 1270–1279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

