Inhibition of SypG-induced biofilms and host colonization by the negative regulator SypE in Vibrio fischeri
- PMID: 23555890
- PMCID: PMC3610818
- DOI: 10.1371/journal.pone.0060076
Inhibition of SypG-induced biofilms and host colonization by the negative regulator SypE in Vibrio fischeri
Abstract
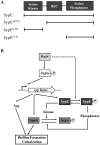
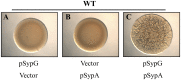
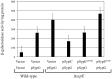
Vibrio fischeri produces a specific biofilm to promote colonization of its eukaryotic host, the squid Euprymna scolopes. Formation of this biofilm is induced by the sensor kinase RscS, which functions upstream of the response regulator SypG to regulate transcription of the symbiosis polysaccharide (syp) locus. Biofilm formation is also controlled by SypE, a multi-domain response regulator that consists of a central regulatory receiver (REC) domain flanked by an N-terminal serine kinase domain and a C-terminal serine phosphatase domain. SypE permits biofilm formation under rscS overexpression conditions, but inhibits biofilms induced by overexpression of sypG. We previously investigated the function of SypE in controlling biofilm formation induced by RscS. Here, we examined the molecular mechanism by which SypE naturally inhibits SypG-induced biofilms. We found that SypE's N-terminal kinase domain was both required and sufficient to inhibit SypG-induced biofilms. This effect did not occur at the level of syp transcription. Instead, under sypG-overexpressing conditions, SypE inhibited biofilms by promoting the phosphorylation of another syp regulator, SypA, a putative anti-sigma factor antagonist. Inhibition by SypE of SypG-induced biofilm formation could be overcome by the expression of a non-phosphorylatable SypA mutant, indicating that SypE functions primarily if not exclusively to control SypA activity via phosphorylation. Finally, the presence of SypE was detrimental to colonization under sypG-overexpressing conditions, as cells deleted for sypE outcompeted wild-type cells for colonization when both strains overexpressed sypG. These results provide further evidence that biofilm formation is critical to symbiotic colonization, and support a model in which SypE naturally functions to restrict biofilm formation, and thus host colonization, to the appropriate environmental conditions.
Conflict of interest statement
Figures





Similar articles
-
Inactivation of a novel response regulator is necessary for biofilm formation and host colonization by Vibrio fischeri.Mol Microbiol. 2011 Oct;82(1):114-30. doi: 10.1111/j.1365-2958.2011.07800.x. Epub 2011 Aug 30. Mol Microbiol. 2011. PMID: 21854462 Free PMC article.
-
The putative hybrid sensor kinase SypF coordinates biofilm formation in Vibrio fischeri by acting upstream of two response regulators, SypG and VpsR.J Bacteriol. 2008 Jul;190(14):4941-50. doi: 10.1128/JB.00197-08. Epub 2008 May 9. J Bacteriol. 2008. PMID: 18469094 Free PMC article.
-
A single point mutation is sufficient to drive syp-dependent biofilm formation and promote colonization by Vibrio fischeri.J Bacteriol. 2025 Aug 21;207(8):e0013125. doi: 10.1128/jb.00131-25. Epub 2025 Jul 14. J Bacteriol. 2025. PMID: 40657914 Free PMC article.
-
An intricate network of regulators controls biofilm formation and colonization by Vibrio fischeri.Mol Microbiol. 2009 Nov;74(4):782-9. doi: 10.1111/j.1365-2958.2009.06899.x. Epub 2009 Oct 8. Mol Microbiol. 2009. PMID: 19818022 Free PMC article. Review.
-
Control of biofilm formation and colonization in Vibrio fischeri: a role for partner switching?Environ Microbiol. 2010 Aug;12(8):2051-9. doi: 10.1111/j.1462-2920.2010.02269.x. Epub 2010 Jun 9. Environ Microbiol. 2010. PMID: 21966901 Free PMC article. Review.
Cited by
-
Engineering Vibrio fischeri for Inducible Gene Expression.Open Microbiol J. 2014 Oct 31;8:122-9. doi: 10.2174/1874285801408010122. eCollection 2014. Open Microbiol J. 2014. PMID: 25408777 Free PMC article.
-
Corrigendum: Assessing the function of STAS domain protein SypA in Vibrio fischeri using a comparative analysis.Front Microbiol. 2017 Apr 26;8:735. doi: 10.3389/fmicb.2017.00735. eCollection 2017. Front Microbiol. 2017. PMID: 28450861 Free PMC article.
-
Transcriptional pathways across colony biofilm models in the symbiont Vibrio fischeri.mSystems. 2024 Jan 23;9(1):e0081523. doi: 10.1128/msystems.00815-23. Epub 2023 Dec 21. mSystems. 2024. PMID: 38126773 Free PMC article.
-
Identification of a novel matrix protein that promotes biofilm maturation in Vibrio fischeri.J Bacteriol. 2015 Feb;197(3):518-28. doi: 10.1128/JB.02292-14. Epub 2014 Nov 17. J Bacteriol. 2015. PMID: 25404700 Free PMC article.
-
Computational and cellular exploration of the protein-protein interaction between Vibrio fischeri STAS domain protein SypA and serine kinase SypE.Commun Integr Biol. 2023 Apr 20;16(1):2203626. doi: 10.1080/19420889.2023.2203626. eCollection 2023. Commun Integr Biol. 2023. PMID: 37091830 Free PMC article.
References
-
- Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2: 95–108. - PubMed
-
- Hogan D, Kolter R (2002) Why are bacteria refractory to antimicrobials? Curr Opin Microbiol 5: 472–477. - PubMed
-
- Kuchma SL, O’Toole GA (2000) Surface-induced and biofilm-induced changes in gene expression. Curr Opin Biotechnol 11: 429–433. - PubMed
-
- Yip ES, Grublesky BT, Hussa EA, Visick KL (2005) A novel, conserved cluster of genes promotes symbiotic colonization and sigma-dependent biofilm formation by Vibrio fischeri . Mol Microbiol 57: 1485–1498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

