A novel role of the Sp/KLF transcription factor KLF11 in arresting progression of endometriosis
- PMID: 23555910
- PMCID: PMC3610699
- DOI: 10.1371/journal.pone.0060165
A novel role of the Sp/KLF transcription factor KLF11 in arresting progression of endometriosis
Abstract
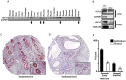
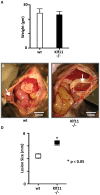
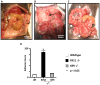
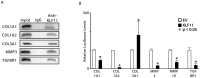
Endometriosis affects approximately 10% of young, reproductive-aged women. Disease associated pelvic pain; infertility and sexual dysfunction have a significant adverse clinical, social and financial impact. As precise disease etiology has remained elusive, current therapeutic strategies are empiric, unfocused and often unsatisfactory. Lack of a suitable genetic model has impaired further translational research in the field. In this study, we evaluated the role of the Sp/KLF transcription factor KLF11/Klf11 in the pathogenesis of endometriosis. KLF11, a human disease-associated gene is etiologically implicated in diabetes, uterine fibroids and cancer. We found that KLF11 expression was diminished in human endometriosis implants and further investigated its pathogenic role in Klf11-/- knockout mice with surgically induced endometriotic lesions. Lesions in Klf11-/- animals were large and associated with prolific fibrotic adhesions resembling advanced human disease in contrast to wildtype controls. To determine phenotype-specificity, endometriosis was also generated in Klf9-/- animals. Unlike in Klf11-/- mice, lesions in Klf9-/- animals were neither large, nor associated with a significant fibrotic response. KLF11 also bound to specific elements located in the promoter regions of key fibrosis-related genes from the Collagen, MMP and TGF-β families in endometrial stromal cells. KLF11 binding resulted in transcriptional repression of these genes. In summary, we identify a novel pathogenic role for KLF11 in preventing de novo disease-associated fibrosis in endometriosis. Our model validates in vivo the phenotypic consequences of dysregulated Klf11 signaling. Additionally, it provides a robust means not only for further detailed mechanistic investigation but also the ability to test any emergent translational ramifications thereof, so as to expand the scope and capability for treatment of endometriosis.
Conflict of interest statement
Figures



References
-
- Eskenazi B, Warner ML (1997) Epidemiology of endometriosis. Obstet Gynecol Clin North Am 24: 235–258. - PubMed
-
- Giudice LC, Kao LC (2004) Endometriosis. Lancet 364: 1789–1799. - PubMed
-
- Balasch J, Creus M, Fabregues F, Carmona F, Ordi J, et al. (1996) Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 11: 387–391. - PubMed
-
- Carter JE (1994) Laparoscopic Treatment for Chronic Pelvic Pain: Results from Three-Year Follow-up. J Am Assoc Gynecol Laparosc 1: S6–7. - PubMed
-
- Barnhart K, Dunsmoor-Su R, Coutifaris C (2002) Effect of endometriosis on in vitro fertilization. Fertil Steril 77: 1148–1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

