Using multivalent adenoviral vectors for HIV vaccination
- PMID: 23555957
- PMCID: PMC3610663
- DOI: 10.1371/journal.pone.0060347
Using multivalent adenoviral vectors for HIV vaccination
Abstract
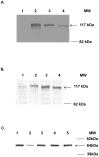
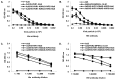
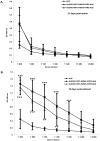
Adenoviral vectors have been used for a variety of vaccine applications including cancer and infectious diseases. Traditionally, Ad-based vaccines are designed to express antigens through transgene expression of a given antigen. For effective vaccine development it is often necessary to express or present multiple antigens to the immune system to elicit an optimal vaccine as observed preclinically with mosaic/polyvalent HIV vaccines or malaria vaccines. Due to the wide flexibility of Ad vectors they are an ideal platform for expressing large amounts of antigen and/or polyvalent mosaic antigens. Ad vectors that display antigens on their capsid surface can elicit a robust humoral immune response, the "antigen capsid-incorporation" strategy. The adenoviral hexon protein has been utilized to display peptides in the majority of vaccine strategies involving capsid incorporation. Based on our abilities to manipulate hexon HVR2 and HVR5, we sought to manipulate HVR1 in the context of HIV antigen display for the first time ever. More importantly, peptide incorporation within HVR1 was utilized in combination with other HVRs, thus creating multivalent vectors. To date this is the first report where dual antigens are displayed within one Ad hexon particle. These vectors utilize HVR1 as an incorporation site for a seven amino acid region of the HIV glycoprotein 41, in combination with six Histidine incorporation within HVR2 or HVR5. Our study illustrates that these multivalent antigen vectors are viable and can present HIV antigen as well as His6 within one Ad virion particle. Furthermore, mouse immunizations with these vectors demonstrate that these vectors can elicit a HIV and His6 epitope-specific humoral immune response.
Conflict of interest statement
Figures



References
-
- Crompton J, Toogood CI, Wallis N, Hay RT (1994) Expression of a foreign epitope on the surface of the adenovirus hexon. JGenVirol 75 (Pt 1): 133–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

