Myocardial remodeling in diabetic cardiomyopathy associated with cardiac mast cell activation
- PMID: 23556005
- PMCID: PMC3612033
- DOI: 10.1371/journal.pone.0060827
Myocardial remodeling in diabetic cardiomyopathy associated with cardiac mast cell activation
Retraction in
-
Retraction: Myocardial Remodeling in Diabetic Cardiomyopathy Associated with Cardiac Mast Cell Activation.PLoS One. 2024 Jun 11;19(6):e0305584. doi: 10.1371/journal.pone.0305584. eCollection 2024. PLoS One. 2024. PMID: 38861555 Free PMC article. No abstract available.
Abstract
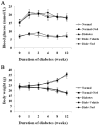
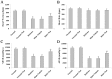
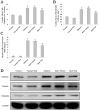
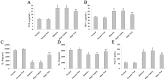
Diabetic cardiomyopathy is a specific disease process distinct from coronary artery disease and hypertension. The disease features cardiac remodeling stimulated by hyperglycemia of the left ventricle wall and disrupts contractile functions. Cardiac mast cells may be activated by metabolic byproducts resulted from hyperglycermia and then participate in the remodeling process by releasing a multitude of cytokines and bioactive enzymes. Nedocromil, a pharmacologic stabilizer of mast cells, has been shown to normalize cytokine levels and attenuate cardiac remodeling. In this study, we describe the activation of cardiac mast cells by inducing diabetes in normal mice using streptozotocin (STZ). Next, we treated the diabetic mice with nedocromil for 12 weeks and then examined their hearts for signs of cardiac remodeling and quantified contractile function. We observed significantly impaired heart function in diabetic mice, as well as increased cardiac mast cell density and elevated mast cell secretions that correlated with gene expression and aberrant cytokine levels associated with cardiac remodeling. Nedocromil treatment halted contractile dysfunction in diabetic mice and reduced cardiac mast cell density, which correlated with reduced bioactive enzyme secretions, reduced expression of extracellular matrix remodeling factors and collagen synthesis, and normalized cytokine levels. However, the results showed nedocromil treatments did not return diabetic mice to a normal state. We concluded that manipulation of cardiac mast cell function is sufficient to attenuate cardiomyopathy stimulated by diabetes, but other cellular pathways also contribute to the disease process.
Conflict of interest statement
Figures

Similar articles
-
MOTS-c Peptide Attenuated Diabetic Cardiomyopathy in STZ-Induced Type 1 Diabetic Mouse Model.Cardiovasc Drugs Ther. 2025 Jun;39(3):491-498. doi: 10.1007/s10557-023-07540-2. Epub 2023 Dec 23. Cardiovasc Drugs Ther. 2025. PMID: 38141139
-
H3 Relaxin Protects Against Myocardial Injury in Experimental Diabetic Cardiomyopathy by Inhibiting Myocardial Apoptosis, Fibrosis and Inflammation.Cell Physiol Biochem. 2017;43(4):1311-1324. doi: 10.1159/000481843. Epub 2017 Oct 9. Cell Physiol Biochem. 2017. PMID: 28992627
-
Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid.Cardiovasc Diabetol. 2012 Jun 19;11:73. doi: 10.1186/1475-2840-11-73. Cardiovasc Diabetol. 2012. PMID: 22713251 Free PMC article.
-
[Diabetic cardiomyopathy].Sheng Li Ke Xue Jin Zhan. 2010 Feb;41(1):31-6. Sheng Li Ke Xue Jin Zhan. 2010. PMID: 21417012 Review. Chinese.
-
The immunology of diabetic cardiomyopathy.Front Endocrinol (Lausanne). 2025 Apr 7;16:1542208. doi: 10.3389/fendo.2025.1542208. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40260277 Free PMC article. Review.
Cited by
-
Astragalus polysaccharides protect cardiac stem and progenitor cells by the inhibition of oxidative stress-mediated apoptosis in diabetic hearts.Drug Des Devel Ther. 2018 Apr 20;12:943-954. doi: 10.2147/DDDT.S155686. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29719380 Free PMC article.
-
Mast Cells in Diabetes and Diabetic Wound Healing.Adv Ther. 2020 Nov;37(11):4519-4537. doi: 10.1007/s12325-020-01499-4. Epub 2020 Sep 15. Adv Ther. 2020. PMID: 32935286 Free PMC article. Review.
-
Substance P-mediated cardiac mast cell activation: An in vitro study.Neuropeptides. 2019 Apr;74:52-59. doi: 10.1016/j.npep.2019.01.002. Epub 2019 Jan 8. Neuropeptides. 2019. PMID: 30660328 Free PMC article.
-
Inhibition of JNK by compound C66 prevents pathological changes of the aorta in STZ-induced diabetes.J Cell Mol Med. 2014 Jun;18(6):1203-12. doi: 10.1111/jcmm.12267. Epub 2014 Apr 10. J Cell Mol Med. 2014. PMID: 24720784 Free PMC article.
-
Established and Emerging Roles of Epigenetic Regulation in Diabetic Cardiomyopathy.Diabetes Metab Res Rev. 2025 Sep;41(6):e70081. doi: 10.1002/dmrr.70081. Diabetes Metab Res Rev. 2025. PMID: 40831067 Free PMC article. Review.
References
-
- Liu JW, Liu D, Cui KZ, Xu Y, Li YB, et al. (2012) Recent advances in understanding the biochemical and molecular mechanism of diabetic cardiomyopathy. Biochem Biophys Res Commun 427: 441–443. - PubMed
-
- Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME (2008) Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 121: 748–757. - PubMed
-
- Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, et al. (2002) Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 346: 1699–1705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

