The rise, fall and subsequent triumph of thalidomide: lessons learned in drug development
- PMID: 23556097
- PMCID: PMC3573415
- DOI: 10.1177/2040620711413165
The rise, fall and subsequent triumph of thalidomide: lessons learned in drug development
Abstract
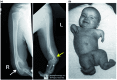
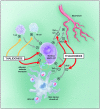
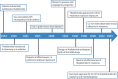
Perhaps no other drug in modern medicine rivals the dramatic revitalization of thalidomide. Originally marketed as a sedative, thalidomide gained immense popularity worldwide among pregnant women because of its effective anti-emetic properties in morning sickness. Mounting evidence of human teratogenicity marked a dramatic fall from grace and led to widespread social, legal and economic ramifications. Despite its tragic past thalidomide emerged several decades later as a novel and highly effective agent in the treatment of various inflammatory and malignant diseases. In 2006 thalidomide completed its remarkable renaissance becoming the first new agent in over a decade to gain approval for the treatment of plasma cell myeloma. The catastrophic collapse yet subsequent revival of thalidomide provides important lessons in drug development. Never entirely abandoned by the medical community, thalidomide resurfaced as an important drug once the mechanisms of action were further studied and better understood. Ongoing research and development of related drugs such as lenalidomide now represent a class of irreplaceable drugs in hematological malignancies. Further, the tragedies associated with this agent stimulated the legislation which revamped the FDA regulatory process, expanded patient informed consent procedures and mandated more transparency from drug manufacturers. Finally, we review recent clinical trials summarizing selected medical indications for thalidomide with an emphasis on hematologic malignancies. Herein, we provide a historic perspective regarding the up-and-down development of thalidomide. Using PubMed databases we conducted searches using thalidomide and associated keywords highlighting pharmacology, mechanisms of action, and clinical uses.
Keywords: anti-angiogenic; anti-apoptosis; graft-versus-host disease; plasma cell myeloma; teratogenic.
Conflict of interest statement
Professor Lazarus has been a speaker for Celgene Corporation.
Figures


References
-
- Anagnostopoulos A., Weber D., Rankin K., Delasalle K., Alexanian R. (2003) Thalidomide and dexamethasone for resistant multiple myeloma. Br J Haematol 121: 768–771 - PubMed
-
- Arora M., Wagner J.E., Davies S.M., Blazar B.R., Defor T., Enright H., et al. (2001) Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant 7: 265–273 - PubMed
-
- Auwerda J.J., Sonneveld P., de Maat M.P., Leebeek F.W. (2007) Prothrombotic coagulation abnormalities in patients with newly diagnosed multiple myeloma. Haematologica 92: 279–280 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

