Silver(I) complex formation with cysteine, penicillamine, and glutathione
- PMID: 23556419
- PMCID: PMC3684387
- DOI: 10.1021/ic400192c
Silver(I) complex formation with cysteine, penicillamine, and glutathione
Abstract
The complex formation between silver(I) and cysteine (H2Cys), penicillamine (H2Pen), and glutathione (H3Glu) in alkaline aqueous solution was examined using extended X-ray absorption fine structure (EXAFS) and (109)Ag NMR spectroscopic techniques. The complexes formed in 0.1 mol dm(-3) Ag(I) solutions with cysteine and penicillamine were investigated for ligand/Ag(I) (L/Ag) mole ratios increasing from 2.0 to 10.0. For the series of cysteine solutions (pH 10-11) a mean Ag-S bond distance of 2.45 ± 0.02 Å consistently emerged, while for penicillamine (pH 9) the average Ag-S bond distance gradually increased from 2.40 to 2.44 ± 0.02 Å. EXAFS and (109)Ag NMR spectra of a concentrated Ag(I)-cysteine solution (C(Ag(I)) = 0.8 mol dm(-3), L/Ag = 2.2) showed a mean Ag-S bond distance of 2.47 ± 0.02 Å and δ((109)Ag) 1103 ppm, consistent with prevailing, partially oligomeric AgS3 coordinated species, while for penicillamine (C(Ag(I)) = 0.5 mol dm(-3), L/Ag = 2.0) the mean Ag-S bond distance of 2.40 ± 0.02 Å and δ((109)Ag) 922 ppm indicate that mononuclear AgS2 coordinated complexes dominate. For Ag(I)-glutathione solutions (C(Ag(I)) = 0.01 mol dm(-3), pH ∼11), mononuclear AgS2 coordinated species with a mean Ag-S bond distance of 2.36 ± 0.02 Å dominate for L/Ag mole ratios from 2.0 to 10.0. The crystal structure of the silver(I)-cysteine compound (NH4)Ag2(HCys)(Cys)·H2O (1) precipitating at pH ∼10 was solved and showed a layer structure with both AgS3 and AgS3N coordination to the cysteinate ligands. A redetermination of the crystal structure of Ag(HPen)·H2O (2) confirmed the proposed digonal AgS2 coordination environment to bridging thiolate sulfur atoms in polymeric intertwining chains forming a double helix. A survey of Ag-S bond distances for crystalline Ag(I) complexes with S-donor ligands in different AgS2, AgS2(O/N), and AgS3 coordination environments was used, together with a survey of (109)Ag NMR chemical shifts, to assist assignments of the Ag(I) coordination in solution.
Figures

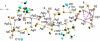
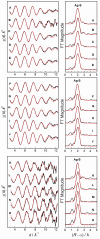
 ; Fit
; Fit  ) are presented in Table 5 (labelled with *).
) are presented in Table 5 (labelled with *).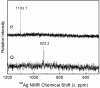
Similar articles
-
Cadmium(II) complex formation with cysteine and penicillamine.Inorg Chem. 2009 Jul 6;48(13):5758-71. doi: 10.1021/ic802278r. Inorg Chem. 2009. PMID: 19469490 Free PMC article.
-
Mercury(II) complex formation with glutathione in alkaline aqueous solution.J Biol Inorg Chem. 2008 May;13(4):541-53. doi: 10.1007/s00775-008-0342-2. J Biol Inorg Chem. 2008. PMID: 18224359
-
XAS Investigation of Silver(I) Coordination in Copper(I) Biological Binding Sites.Inorg Chem. 2015 Dec 21;54(24):11688-96. doi: 10.1021/acs.inorgchem.5b01658. Epub 2015 Dec 3. Inorg Chem. 2015. PMID: 26632864
-
Lead(II) complex formation with L-cysteine in aqueous solution.Inorg Chem. 2015 Mar 2;54(5):2160-70. doi: 10.1021/ic5025668. Epub 2015 Feb 19. Inorg Chem. 2015. PMID: 25695880 Free PMC article.
-
Lead(II) complex formation with glutathione.Inorg Chem. 2012 Jun 4;51(11):6285-98. doi: 10.1021/ic300496t. Epub 2012 May 17. Inorg Chem. 2012. PMID: 22594853 Free PMC article.
Cited by
-
Revisiting Fluoride in the Twenty-First Century: Safety and Efficacy Considerations.Front Oral Health. 2022 Jul 4;3:873157. doi: 10.3389/froh.2022.873157. eCollection 2022. Front Oral Health. 2022. PMID: 35860375 Free PMC article.
-
Circularly polarised luminescence in an RNA-based homochiral, self-repairing, coordination polymer hydrogel.J Mater Chem C Mater. 2022 Apr 20;10(18):7329-7335. doi: 10.1039/d2tc00366j. eCollection 2022 May 12. J Mater Chem C Mater. 2022. PMID: 35706420 Free PMC article.
-
Sequential, low-temperature aqueous synthesis of Ag-In-S/Zn quantum dots via staged cation exchange under biomineralization conditions.J Mater Chem B. 2022 Jun 22;10(24):4529-4545. doi: 10.1039/d2tb00682k. J Mater Chem B. 2022. PMID: 35608268 Free PMC article.
-
Crystal structure of catena-poly[silver(I)-μ-l-valinato-κ2N:O].Acta Crystallogr E Crystallogr Commun. 2017 Feb 14;73(Pt 3):354-357. doi: 10.1107/S2056989017001815. eCollection 2017 Mar 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 28316807 Free PMC article.
-
Assessing the influence of microwave-assisted synthesis parameters and stabilizing ligands on the optical properties of AIS/ZnS quantum dots.Sci Rep. 2022 Dec 20;12(1):22000. doi: 10.1038/s41598-022-25498-3. Sci Rep. 2022. PMID: 36539585 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

