Dose- and time-dependent neuroprotective effects of Pycnogenol following traumatic brain injury
- PMID: 23557184
- PMCID: PMC3751204
- DOI: 10.1089/neu.2013.2910
Dose- and time-dependent neuroprotective effects of Pycnogenol following traumatic brain injury
Abstract
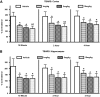
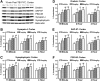
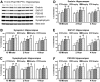
After traumatic brain injury (TBI), both primary and secondary injury cascades are initiated, leading to neuronal death and cognitive dysfunction. We have previously shown that the combinational bioflavonoid, Pycnogenol (PYC), alters some secondary injury cascades and protects synaptic proteins when administered immediately following trauma. The purpose of the present study was to explore further the beneficial effects of PYC and to test whether it can be used in a more clinically relevant fashion. Young adult male Sprague-Dawley rats were subjected to a unilateral moderate/severe cortical contusion. Subjects received a single intravenous (i.v.) injection of PYC (1, 5, or 10 mg/kg) or vehicle, with treatment initiated at 15 min, 2 h, or 4 h post injury. All rats were killed at 96 h post TBI. Both the cortex and hippocampus ipsilateral and contralateral to the injury were evaluated for possible changes in oxidative stress (thiobarbituric acid reactive species; TBARS) and both pre- and post-synaptic proteins (synapsin-I, synaptophysin, drebrin, post synaptic density protein-95, and synapse associated protein-97). Following TBI, TBARS were significantly increased in both the injured cortex and ipsilateral hippocampus. Regardless of the dose and delay in treatment, PYC treatment significantly lowered TBARS. PYC treatment significantly protected both the cortex and hippocampus from injury-related declines in pre- and post-synaptic proteins. These results demonstrate that a single i.v. treatment of PYC is neuroprotective after TBI with a therapeutic window of at least 4 h post trauma. The natural bioflavonoid PYC may provide a possible therapeutic intervention in neurotrauma.
Figures
References
-
- Faul M. Xu L. Wald M. Coronado V. Atlanta, GA: Centers for Disease control and Prevention National Center for Injury Prevention and Control; 2010. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006.
-
- Baldwin SA. Gibson T. Callihan CT. Sullivan PG. Palmer E. Scheff SW. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical disector method for cell counting. J Neurotrauma. 1997;14:385–398. - PubMed
-
- Hovda DA. Yoshino A. Kawamata T. Katayama Y. Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: A cytochrome oxidase histochemistry study. Brain Res. 1991;567:1–10. - PubMed
-
- Kawamata T. Katayama Y. Hovda DA. Yoshino A. Becker DP. Lactate accumulation following concussive brain injury: The role of ionic fluxes induced by excitatory amino acids. Brain Res. 1995;674:196–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

