Radiation mitigating properties of the lignan component in flaxseed
- PMID: 23557217
- PMCID: PMC3636021
- DOI: 10.1186/1471-2407-13-179
Radiation mitigating properties of the lignan component in flaxseed
Abstract
Background: Wholegrain flaxseed (FS), and its lignan component (FLC) consisting mainly of secoisolariciresinol diglucoside (SDG), have potent lung radioprotective properties while not abrogating the efficacy of radiotherapy. However, while the whole grain was recently shown to also have potent mitigating properties in a thoracic radiation pneumonopathy model, the bioactive component in the grain responsible for the mitigation of lung damage was never identified. Lungs may be exposed to radiation therapeutically for thoracic malignancies or incidentally following detonation of a radiological dispersion device. This could potentially lead to pulmonary inflammation, oxidative tissue injury, and fibrosis. This study aimed to evaluate the radiation mitigating effects of FLC in a mouse model of radiation pneumonopathy.
Methods: We evaluated FLC-supplemented diets containing SDG lignan levels comparable to those in 10% and 20% whole grain diets. 10% or 20% FLC diets as compared to an isocaloric control diet (0% FLC) were given to mice (C57/BL6) (n=15-30 mice/group) at 24, 48, or 72-hours after single-dose (13.5 Gy) thoracic x-ray treatment (XRT). Mice were evaluated 4 months post-XRT for blood oxygenation, lung inflammation, fibrosis, cytokine and oxidative damage levels, and survival.
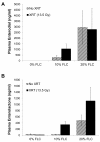
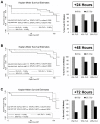
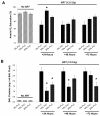
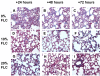
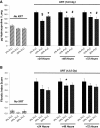
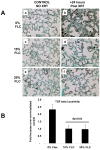
Results: FLC significantly mitigated radiation-related animal death. Specifically, mice fed 0% FLC demonstrated 36.7% survival 4 months post-XRT compared to 60-73.3% survival in mice fed 10%-20% FLC initiated 24-72 hours post-XRT. FLC also mitigated radiation-induced lung fibrosis whereby 10% FLC initiated 24-hours post-XRT significantly decreased fibrosis as compared to mice fed control diet while the corresponding TGF-beta1 levels detected immunohistochemically were also decreased. Additionally, 10-20% FLC initiated at any time point post radiation exposure, mitigated radiation-induced lung injury evidenced by decreased bronchoalveolar lavage (BAL) protein and inflammatory cytokine/chemokine release at 16 weeks post-XRT. Importantly, neutrophilic and overall inflammatory cell infiltrate in airways and levels of nitrotyrosine and malondialdehyde (protein and lipid oxidation, respectively) were also mitigated by the lignan diet.
Conclusions: Dietary FLC given early post-XRT mitigated radiation effects by decreasing inflammation, lung injury and eventual fibrosis while improving survival. FLC may be a useful agent, mitigating adverse effects of radiation in individuals exposed to incidental radiation, inhaled radioisotopes or even after the initiation of radiation therapy to treat malignancy.
Figures




References
-
- Chin FK. Scenario of a dirty bomb in an urban environment and acute management of radiation poisoning and injuries. Singapore Med J. 2007;48(10):950–957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

