A C. elegans model to study human metabolic regulation
- PMID: 23557393
- PMCID: PMC3636097
- DOI: 10.1186/1743-7075-10-31
A C. elegans model to study human metabolic regulation
Abstract
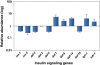
Lipid metabolic disorder is a critical risk factor for metabolic syndrome, triggering debilitating diseases like obesity and diabetes. Both obesity and diabetes are the epicenter of important medical issues, representing a major international public health threat. Accumulation of fat in adipose tissue, muscles and liver and/or the defects in their ability to metabolize fatty acids, results in insulin resistance. This triggers an early pathogenesis of type 2 diabetes (T2D). In mammals, lipid metabolism involves several organs, including the brain, adipose tissue, muscles, liver, and gut. These organs are part of complex homeostatic system and communicate through hormones, neurons and metabolites. Our study dissects the importance of mammalian Krüppel-like factors in over all energy homeostasis. Factors controlling energy metabolism are conserved between mammals and Caenorhabditis elegans providing a new and powerful strategy to delineate the molecular pathways that lead to metabolic disorder. The C. elegans intestine is our model system where genetics, molecular biology, and cell biology are used to identify and understand genes required in fat metabolism. Thus far, we have found an important role of C. elegans KLF in FA biosynthesis, mitochondrial proliferation, lipid secretion, and β-oxidation. The mechanism by which KLF controls these events in lipid metabolism is unknown. We have recently observed that C. elegans KLF-3 selectively acts on insulin components to regulate insulin pathway activity. There are many factors that control energy homeostasis and defects in this control system are implicated in the pathogenesis of human obesity and diabetes. In this review we are discussing a role of KLF in human metabolic regulation.
Figures

References
-
- Schwartz MW, Woods SC, Porte DR Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

