Carbofluoresceins and carborhodamines as scaffolds for high-contrast fluorogenic probes
- PMID: 23557713
- PMCID: PMC3691720
- DOI: 10.1021/cb4000822
Carbofluoresceins and carborhodamines as scaffolds for high-contrast fluorogenic probes
Abstract
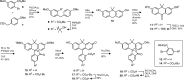
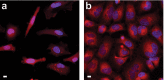
Fluorogenic molecules are important tools for advanced biochemical and biological experiments. The extant collection of fluorogenic probes is incomplete, however, leaving regions of the electromagnetic spectrum unutilized. Here, we synthesize green-excited fluorescent and fluorogenic analogues of the classic fluorescein and rhodamine 110 fluorophores by replacement of the xanthene oxygen with a quaternary carbon. These anthracenyl "carbofluorescein" and "carborhodamine 110" fluorophores exhibit excellent fluorescent properties and can be masked with enzyme- and photolabile groups to prepare high-contrast fluorogenic molecules useful for live cell imaging experiments and super-resolution microscopy. Our divergent approach to these red-shifted dye scaffolds will enable the preparation of numerous novel fluorogenic probes with high biological utility.
Figures



References
-
- Haugland R. P., Spence M. T. Z., Johnson I. D., and Basey A. (2005) The Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 10th ed., Molecular Probes, Eugene, OR.
-
- Grimm J. B.; Heckman L. M.; Lavis L. D. (2013) The chemistry of small-molecule fluorogenic probes. Prog. Mol. Biol. Transl. Sci. 113, 1–34. - PubMed
-
- Gee K. R.; Sun W.-C.; Bhalgat M. K.; Upson R. H.; Klaubert D. H.; Latham K. A.; Haugland R. P. (1999) Fluorogenic substrates based on fluorinated umbelliferones for continuous assays of phosphatases and β-galactosidases. Anal. Biochem. 273, 41–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

