Spatial transcriptional profile of the chick and mouse endocardial cushions identify novel regulators of endocardial EMT in vitro
- PMID: 23557753
- PMCID: PMC3659811
- DOI: 10.1016/j.yjmcc.2013.03.016
Spatial transcriptional profile of the chick and mouse endocardial cushions identify novel regulators of endocardial EMT in vitro
Abstract
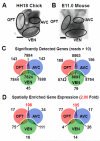
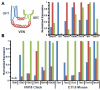
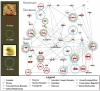
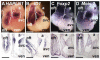
Valvular Interstitial Cells (VICs) are a common substrate for congenital and adult heart disease yet the signaling mechanisms governing their formation during early valvulogenesis are incompletely understood. We developed an unbiased strategy to identify genes important in endocardial epithelial-to-mesenchymal transformation (EMT) using a spatial transcriptional profile. Endocardial cells overlaying the cushions of the atrioventricular canal (AVC) and outflow tract (OFT) undergo an EMT to yield VICs. RNA sequencing (RNA-seq) analysis of gene expression between AVC, OFT, and ventricles (VEN) isolated from chick and mouse embryos at comparable stages of development (chick HH18; mouse E11.0) was performed. EMT occurs in the AVC and OFT cushions, but not VEN at this time. 198 genes in the chick (n=1) and 105 genes in the mouse (n=2) were enriched 2-fold in the cushions. Gene regulatory networks (GRN) generated from cushion-enriched gene lists confirmed TGFβ as a nodal point and identified NF-κB as a potential node. To reveal previously unrecognized regulators of EMT four candidate genes, Hapln1, Id1, Foxp2, and Meis2, and a candidate pathway, NF-κB, were selected. In vivo spatial expression of each gene was confirmed by in situ hybridization and a functional role for each in endocardial EMT was determined by siRNA knockdown in a collagen gel assay. Our spatial-transcriptional profiling strategy yielded gene lists which reflected the known biology of the system. Further analysis accurately identified and validated previously unrecognized novel candidate genes and the NF-κB pathway as regulators of endocardial cell EMT in vitro.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Loss of muscleblind-like 1 promotes invasive mesenchyme formation in endocardial cushions by stimulating autocrine TGFβ3.BMC Dev Biol. 2012 Aug 6;12:22. doi: 10.1186/1471-213X-12-22. BMC Dev Biol. 2012. PMID: 22866814 Free PMC article.
-
Fibulin-1 suppresses endothelial to mesenchymal transition in the proximal outflow tract.Mech Dev. 2015 May;136:123-32. doi: 10.1016/j.mod.2014.12.005. Epub 2015 Jan 6. Mech Dev. 2015. PMID: 25575930 Free PMC article.
-
Muscleblind-like 1 is required for normal heart valve development in vivo.BMC Dev Biol. 2015 Oct 15;15:36. doi: 10.1186/s12861-015-0087-4. BMC Dev Biol. 2015. PMID: 26472242 Free PMC article.
-
Nfatc1 directs the endocardial progenitor cells to make heart valve primordium.Trends Cardiovasc Med. 2013 Nov;23(8):294-300. doi: 10.1016/j.tcm.2013.04.003. Epub 2013 May 10. Trends Cardiovasc Med. 2013. PMID: 23669445 Free PMC article. Review.
-
Roles of TGFbeta and BMP during valvulo-septal endocardial cushion formation.Anat Sci Int. 2009 Sep;84(3):77-87. doi: 10.1007/s12565-009-0027-0. Epub 2009 Mar 14. Anat Sci Int. 2009. PMID: 19288174 Review.
Cited by
-
Concordant and Heterogeneity of Single-Cell Transcriptome in Cardiac Development of Human and Mouse.Front Genet. 2022 Jun 27;13:892766. doi: 10.3389/fgene.2022.892766. eCollection 2022. Front Genet. 2022. PMID: 35832197 Free PMC article.
-
Transforming growth factor-β1 regulates epithelial-mesenchymal transition in association with cancer stem-like cells in a breast cancer cell line.Int J Clin Exp Med. 2014 Apr 15;7(4):865-72. eCollection 2014. Int J Clin Exp Med. 2014. PMID: 24955155 Free PMC article.
-
Biallelic loss-of-function variants in PLD1 cause congenital right-sided cardiac valve defects and neonatal cardiomyopathy.J Clin Invest. 2021 Mar 1;131(5):e142148. doi: 10.1172/JCI142148. J Clin Invest. 2021. PMID: 33645542 Free PMC article.
-
Single-Cell RNA Sequencing of Human Embryonic Stem Cell Differentiation Delineates Adverse Effects of Nicotine on Embryonic Development.Stem Cell Reports. 2019 Apr 9;12(4):772-786. doi: 10.1016/j.stemcr.2019.01.022. Epub 2019 Feb 28. Stem Cell Reports. 2019. PMID: 30827876 Free PMC article.
-
Spatial transcriptomics reveals novel genes during the remodelling of the embryonic human arterial valves.PLoS Genet. 2023 Nov 27;19(11):e1010777. doi: 10.1371/journal.pgen.1010777. eCollection 2023 Nov. PLoS Genet. 2023. PMID: 38011284 Free PMC article.
References
-
- Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003 Feb;69(1):58–72. - PubMed
-
- Loffredo CA. Epidemiology of cardiovascular malformations: prevalence and risk factors. Am J Med Genet. 2000;97(4):319–25. Winter. - PubMed
-
- Khairy P, Hosn JA, Broberg C, Cook S, Earing M, Gersony D, et al. Multicenter research in adult congenital heart disease. Int J Cardiol. 2008 Sep 26;129(2):155–9. - PubMed
-
- Lencinas A, Tavares AL, Barnett JV, Runyan RB. Collagen gel analysis of epithelial-mesenchymal transition in the embryo heart: an in vitro model system for the analysis of tissue interaction, signal transduction, and environmental effects. Birth Defects Res C Embryo Today. 2012 Dec;93(4):298–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

