The efficacy of nuclease-resistant Chol-siRNA in primary breast tumors following complexation with PLL-PEG(5K)
- PMID: 23557861
- PMCID: PMC3649532
- DOI: 10.1016/j.biomaterials.2013.03.021
The efficacy of nuclease-resistant Chol-siRNA in primary breast tumors following complexation with PLL-PEG(5K)
Abstract
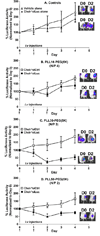
Modifying the sense strand of nuclease-resistant siRNA with 3'-cholesterol (Chol-*siRNA) increases mRNA suppression after i.v. administration but with relatively low efficacy. We previously found evidence in vitro that suggests complexation of Chol-siRNA with PLL-PEG(5K), a block copolymer of poly-l-lysine and 5 kDa polyethylene glycol, may increase the efficacy of Chol-siRNA in vivo in a PLL block length-dependent manner. In this study, the extent that polyplexes of PLL10-PEG(5K), PLL30-PEG(5K), and PLL50-PEG(5K) protect complexed Chol-siRNA in high concentrations of murine serum and affect the activity of Chol-*siRNA in murine 4T1 breast tumor epithelial cells in vitro and in primary orthotopic tumors of 4T1 was compared. PLL-PEG(5K) required 3'-Chol to protect full-length siRNA from nuclease degradation in 90% (v/v) murine serum and protection was increased by increasing PLL block length and nuclease resistance of Chol-siRNA. Polyplexes of Chol-*siLuc suppressed stably expressed luciferase in 4T1-Luc cells to different levels in vitro where PLL30 > PLL50 > PLL10. In contrast, only polyplexes of Chol-*siLuc and PLL30-PEG(5K) or PLL50-PEG(5K) suppressed high levels of luciferase in primary orthotopic tumors of 4T1-Luc after i.v. administration, whereas polyplexes of Chol-*siLuc and PLL10-PEG(5K), inactive Chol-*siCtrl polyplexes of PLL-PEG(5K), or Chol-*siLuc alone had no detectable activity. As a whole, these results indicate that polyplexes of PLL-PEG(5K) increase the efficacy of nuclease-resistant Chol-siRNA in primary breast tumors after i.v. administration in a PLL block length-dependent manner. Thus, complexation of Chol-siRNA with PLL-PEG(5K) may be a promising approach to increase the efficacy of Chol-siRNA in a wide range of primary tumors, metastases, and other tissues but likely requires a PLL block length that balances polymer-related adverse effects, Chol-siRNA bioavailability, and subsequent activity in the target cell.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
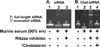


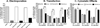

References
-
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. - PubMed
-
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. - PubMed
-
- Bora RS, Gupta D, Mukkur TK, Saini KS. RNA interference therapeutics for cancer: challenges and opportunities (review) Mol Med Report. 2012;6:9–15. - PubMed
-
- Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. - PubMed
-
- Aigner A. Cellular delivery in vivo of siRNA-based therapeutics. Curr Pharm Des. 2008;14:3603–3619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

