Transient receptor potential ankyrin 1 mediates chronic pancreatitis pain in mice
- PMID: 23558009
- PMCID: PMC3680686
- DOI: 10.1152/ajpgi.00005.2013
Transient receptor potential ankyrin 1 mediates chronic pancreatitis pain in mice
Abstract
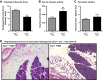
Chronic pancreatitis (CP) is a devastating disease characterized by persistent and uncontrolled abdominal pain. Our lack of understanding is partially due to the lack of experimental models that mimic the human disease and also to the lack of validated behavioral measures of visceral pain. The ligand-gated cation channel transient receptor potential ankyrin 1 (TRPA1) mediates inflammation and pain in early experimental pancreatitis. It is unknown if TRPA1 causes fibrosis and sustained pancreatic pain. We induced CP by injecting the chemical agent trinitrobenzene sulfonic acid (TNBS), which causes severe acute pancreatitis, into the pancreatic duct of C57BL/6 trpa1(+/+) and trpa1(-/-) mice. Chronic inflammatory changes and pain behaviors were assessed after 2-3 wk. TNBS injection caused marked pancreatic fibrosis with increased collagen-staining intensity, atrophy, fatty replacement, monocyte infiltration, and pancreatic stellate cell activation, and these changes were reflected by increased histological damage scores. TNBS-injected animals showed mechanical hypersensitivity during von Frey filament probing of the abdomen, decreased daily voluntary wheel-running activity, and increased immobility scores during open-field testing. Pancreatic TNBS also reduced the threshold to hindpaw withdrawal to von Frey filament probing, suggesting central sensitization. Inflammatory changes and pain indexes were significantly reduced in trpa1(-/-) mice. In conclusion, we have characterized in mice a model of CP that resembles the human condition, with marked histological changes and behavioral measures of pain. We have demonstrated, using novel and objective pain measurements, that TRPA1 mediates inflammation and visceral hypersensitivity in CP and could be a therapeutic target for the treatment of sustained inflammatory abdominal pain.
Keywords: chronic pancreatitis; inflammation; pain; transient receptor potential ankyrin 1; trinitrobenzene sulfonic acid.
Figures





Similar articles
-
TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis.J Neurosci. 2013 Mar 27;33(13):5603-11. doi: 10.1523/JNEUROSCI.1806-12.2013. J Neurosci. 2013. PMID: 23536075 Free PMC article.
-
Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice.Am J Physiol Gastrointest Liver Physiol. 2010 Jan;298(1):G81-91. doi: 10.1152/ajpgi.00221.2009. Epub 2009 Oct 29. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 19875705 Free PMC article.
-
Tanshinone IIA Attenuates Chronic Pancreatitis-Induced Pain in Rats via Downregulation of HMGB1 and TRL4 Expression in the Spinal Cord.Pain Physician. 2015 Jul-Aug;18(4):E615-28. Pain Physician. 2015. PMID: 26218952
-
Modification of the TRP Channel TRPA1 as a Relevant Factor in Migraine-Related Intracranial Hypersensitivity.Int J Mol Sci. 2023 Mar 11;24(6):5375. doi: 10.3390/ijms24065375. Int J Mol Sci. 2023. PMID: 36982450 Free PMC article. Review.
-
Increasingly irritable and close to tears: TRPA1 in inflammatory pain.Cell. 2006 Mar 24;124(6):1123-5. doi: 10.1016/j.cell.2006.03.006. Cell. 2006. PMID: 16564004 Review.
Cited by
-
A Mouse Model for Chronic Pancreatitis via Bile Duct TNBS Infusion.J Vis Exp. 2021 Feb 28;(168):10.3791/62080. doi: 10.3791/62080. J Vis Exp. 2021. PMID: 33720138 Free PMC article.
-
Complement Component 5 Mediates Development of Fibrosis, via Activation of Stellate Cells, in 2 Mouse Models of Chronic Pancreatitis.Gastroenterology. 2015 Sep;149(3):765-76.e10. doi: 10.1053/j.gastro.2015.05.012. Epub 2015 May 19. Gastroenterology. 2015. PMID: 26001927 Free PMC article.
-
A Novel Cellular Therapy to Treat Pancreatic Pain in Experimental Chronic Pancreatitis Using Human Alpha-1 Antitrypsin Overexpressing Mesenchymal Stromal Cells.Biomedicines. 2021 Nov 16;9(11):1695. doi: 10.3390/biomedicines9111695. Biomedicines. 2021. PMID: 34829924 Free PMC article.
-
Towards a neurobiological understanding of pain in chronic pancreatitis: mechanisms and implications for treatment.Pain Rep. 2017 Oct 25;2(6):e625. doi: 10.1097/PR9.0000000000000625. eCollection 2017 Nov. Pain Rep. 2017. PMID: 29392239 Free PMC article. Review.
-
TRPA1 as a therapeutic target for nociceptive pain.Expert Opin Ther Targets. 2020 Oct;24(10):997-1008. doi: 10.1080/14728222.2020.1815191. Epub 2020 Sep 11. Expert Opin Ther Targets. 2020. PMID: 32838583 Free PMC article. Review.
References
-
- Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology 86: 820–828, 1984 - PubMed
-
- Anaparthy R, Pasricha PJ. Pain and chronic pancreatitis: is it the plumbing or the wiring? Curr Gastroenterol Rep 10: 101–106, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

