Arterial insulin resistance in Yucatan micropigs with diet-induced obesity and metabolic syndrome
- PMID: 23558108
- PMCID: PMC3696427
- DOI: 10.1016/j.jdiacomp.2013.02.009
Arterial insulin resistance in Yucatan micropigs with diet-induced obesity and metabolic syndrome
Abstract
Aim: Metabolic syndrome affects a large proportion of the population and increases cardiovascular disease risk. Because metabolic syndrome often co-exists clinically with atherosclerosis, it is difficult to distinguish the respective contributions of the components to vascular abnormalities. Accordingly, we utilized a porcine dietary model of metabolic syndrome without atherosclerosis to investigate early abnormalities of vascular function and signaling.
Methods: Thirty-two Yucatan micropigs were fed either a high-fat, high-simple-sugar, high-calorie (HFHS) or standard chow diet (STD) for 6 months. Neither diet contained added cholesterol. Blood pressure and flow-mediated vasodilatation were assessed at baseline and 6 months. Aortas were harvested at 6 months to assess histology, insulin signaling, and endothelial nitric oxide (eNOS) phosphorylation.
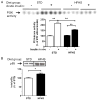
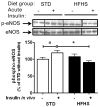
Results: HFHS pigs developed characteristics of metabolic syndrome including obesity, dyslipidemia, and insulin resistance, but without histologic evidence of atherosclerosis. Although arterial intima-media thickness did not differ between groups, vascular dysfunction in HFHS was manifest by increased blood pressure and impaired flow-mediated vasodilation of the femoral artery. Compared with STD, aortas from HFHS exhibited increased p85α expression and Ser307 IRS-1 phosphorylation, and blunted insulin-stimulated IRS-1-associated phosphatidylinositol (PI) 3-kinase activity. In the absence of insulin stimulation, aortic Akt Ser473-phosphorylation was greater in HFHS than in STD. With insulin stimulation, Akt phosphorylation increased in STD, but not HFHS. Insulin-induced Ser1177-phosphorylation of eNOS was decreased in HFHS, compared with STD.
Conclusions: Pigs with metabolic syndrome develop early vascular dysfunction and aortic insulin signaling abnormalities, and could be a useful model for early human vascular abnormalities in this condition.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest that could bias the studies described.
Figures



References
-
- Barbour LA, Mizanoor RS, Gurevich I, Leitner JW, Fischer SJ, Roper MD, et al. Increased P85alpha is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess. J Biol Chem. 2005;280(45):37489–37494. - PubMed
-
- Baron AD. The coupling of glucose metabolism and perfusion in human skeletal muscle. The potential role of endothelium-derived nitric oxide. Diabetes. 1996;45(Suppl 1):S105–S109. - PubMed
-
- Bayturan O, Tuzcu EM, Uno K, Lavoie AJ, Hu T, Shreevatsa A, et al. Comparison of rates of progression of coronary atherosclerosis in patients with diabetes mellitus versus those with the metabolic syndrome. Am J Cardiol. 2010;105(12):1735–1739. - PubMed
-
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949. - PubMed
-
- Bertacca A, Ciccarone A, Cecchetti P, Vianello B, Laurenza I, Maffei M, et al. Continually high insulin levels impair Akt phosphorylation and glucose transport in human myoblasts. Metabolism. 2005;54(12):1687–1693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

