An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells
- PMID: 23558169
- PMCID: PMC3985613
- DOI: 10.1126/science.1236062
An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells
Abstract
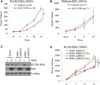
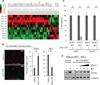
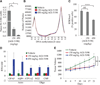
The recent discovery of mutations in metabolic enzymes has rekindled interest in harnessing the altered metabolism of cancer cells for cancer therapy. One potential drug target is isocitrate dehydrogenase 1 (IDH1), which is mutated in multiple human cancers. Here, we examine the role of mutant IDH1 in fully transformed cells with endogenous IDH1 mutations. A selective R132H-IDH1 inhibitor (AGI-5198) identified through a high-throughput screen blocked, in a dose-dependent manner, the ability of the mutant enzyme (mIDH1) to produce R-2-hydroxyglutarate (R-2HG). Under conditions of near-complete R-2HG inhibition, the mIDH1 inhibitor induced demethylation of histone H3K9me3 and expression of genes associated with gliogenic differentiation. Blockade of mIDH1 impaired the growth of IDH1-mutant--but not IDH1-wild-type--glioma cells without appreciable changes in genome-wide DNA methylation. These data suggest that mIDH1 may promote glioma growth through mechanisms beyond its well-characterized epigenetic effects.
Figures
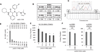
Comment in
-
Therapeutics: targeting an oncometabolite.Nat Rev Cancer. 2013 Jun;13(6):383. doi: 10.1038/nrc3531. Epub 2013 May 3. Nat Rev Cancer. 2013. PMID: 23640211 No abstract available.
-
Cancer. Silencing a metabolic oncogene.Science. 2013 May 3;340(6132):558-9. doi: 10.1126/science.1238523. Science. 2013. PMID: 23641103 Free PMC article.
-
Releasing the block: setting differentiation free with mutant IDH inhibitors.Cancer Cell. 2013 May 13;23(5):570-2. doi: 10.1016/j.ccr.2013.04.024. Cancer Cell. 2013. PMID: 23680144 Free PMC article.
-
Cancer metabolism in breadth and depth.Nat Biotechnol. 2013 Jun;31(6):505-7. doi: 10.1038/nbt.2611. Nat Biotechnol. 2013. PMID: 23752435 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

