Plasmatic ADAMTS-13 metalloprotease and von Willebrand factor in children with cyanotic congenital heart disease
- PMID: 23558858
- PMCID: PMC3854409
- DOI: 10.1590/1414-431x20122603
Plasmatic ADAMTS-13 metalloprotease and von Willebrand factor in children with cyanotic congenital heart disease
Abstract
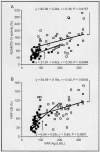
Changes in plasma von Willebrand factor concentration (VWF:Ag) and ADAMTS-13 activity (the metalloprotease that cleaves VWF physiologically) have been reported in several cardiovascular disorders with prognostic implications. We therefore determined the level of these proteins in the plasma of children with cyanotic congenital heart disease (CCHD) undergoing surgical treatment. Forty-eight children were enrolled (age 0.83 to 7.58 years). Measurements were performed at baseline and 48 h after surgery. ELISA, collagen-binding assays and Western blotting were used to estimate antigenic and biological activities, and proteolysis of VWF multimers. Preoperatively, VWF:Ag and ADAMTS-13 activity were decreased (65 and 71% of normal levels considered as 113 (105-129) U/dL and 91 ± 24% respectively, P < 0.003) and correlated (r = 0.39, P = 0.0064). High molecular weight VWF multimers were not related, suggesting an interaction of VWF with cell membranes, followed by proteolytic cleavage. A low preoperative ADAMTS-13 activity, a longer activated partial thromboplastin time and the need for cardiopulmonary bypass correlated with postoperative bleeding (P < 0.05). Postoperatively, ADAMTS-13 activity increased but less extensively than VWF:Ag (respectively, 2.23 and 2.83 times baseline, P < 0.0001), resulting in an increased VWF:Ag/ADAMTS-13 activity ratio (1.20 to 1.54, respectively, pre- and postoperative median values, P = 0.0029). ADAMTS-13 consumption was further confirmed by decreased ADAMTS-13 antigenic concentration (0.91 ± 0.30 to 0.70 ± 0.25 µg/mL, P < 0.0001) and persistent proteolysis of VWF multimers. We conclude that, in pediatric CCHD, changes in circulating ADAMTS-13 suggest enzyme consumption, associated with abnormal structure and function of VWF.
Figures

Similar articles
-
Decreased plasma ADAMTS-13 activity as a predictor of postoperative bleeding in cyanotic congenital heart disease.Clinics (Sao Paulo). 2013 Apr;68(4):531-6. doi: 10.6061/clinics/2013(04)15. Clinics (Sao Paulo). 2013. PMID: 23778350 Free PMC article.
-
Elevated preoperative von Willebrand factor is associated with perioperative thrombosis in infants and neonates with congenital heart disease.J Thromb Haemost. 2017 Dec;15(12):2306-2316. doi: 10.1111/jth.13860. Epub 2017 Oct 26. J Thromb Haemost. 2017. PMID: 28981194
-
Severe dengue is associated with consumption of von Willebrand factor and its cleaving enzyme ADAMTS-13.PLoS Negl Trop Dis. 2012;6(5):e1628. doi: 10.1371/journal.pntd.0001628. Epub 2012 May 1. PLoS Negl Trop Dis. 2012. PMID: 22563509 Free PMC article.
-
Assays of ADAMTS-13 activity.Semin Hematol. 2004 Jan;41(1):41-7. doi: 10.1053/j.seminhematol.2003.10.005. Semin Hematol. 2004. PMID: 14727258 Review.
-
Von Willebrand factor, ADAMTS-13, and thrombotic thrombocytopenic purpura.Semin Thromb Hemost. 2010 Feb;36(1):71-81. doi: 10.1055/s-0030-1248726. Epub 2010 Apr 13. Semin Thromb Hemost. 2010. PMID: 20391298 Review.
Cited by
-
Coagulation Profile in Neonates with Congenital Heart Disease: A Pilot Study.Medicina (Kaunas). 2024 Feb 3;60(2):268. doi: 10.3390/medicina60020268. Medicina (Kaunas). 2024. PMID: 38399555 Free PMC article.
-
The Role of the Coagulation System in Peripheral Arterial Disease: Interactions with the Arterial Wall and Its Vascular Microenvironment and Implications for Rational Therapies.Int J Mol Sci. 2022 Nov 29;23(23):14914. doi: 10.3390/ijms232314914. Int J Mol Sci. 2022. PMID: 36499242 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

