Chemerin connects fat to arterial contraction
- PMID: 23559624
- PMCID: PMC3752465
- DOI: 10.1161/ATVBAHA.113.301476
Chemerin connects fat to arterial contraction
Abstract
Objective: Obesity and hypertension are comorbid in epidemic proportion, yet their biological connection is largely a mystery. The peptide chemerin is a candidate for connecting fat deposits around the blood vessel (perivascular adipose tissue) to arterial contraction. We presently tested the hypothesis that chemerin is expressed in perivascular adipose tissue and is vasoactive, supporting the existence of a chemerin axis in the vasculature.
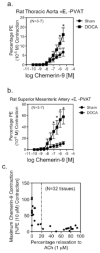
Approach and results: Real-time polymerase chain reaction, immunohistochemistry, and Western analyses supported the synthesis and expression of chemerin in perivascular adipose tissue, whereas the primary chemerin receptor ChemR23 was expressed both in the tunica media and endothelial layer. The ChemR23 agonist chemerin-9 caused receptor, concentration-dependent contraction in the isolated rat thoracic aorta, superior mesenteric artery, and mesenteric resistance artery, and contraction was significantly amplified (more than 100%) when nitric oxide synthase was inhibited and the endothelial cell mechanically removed or tone was placed on the arteries. The novel ChemR23 antagonist CCX832 inhibited phenylephrine-induced and prostaglandin F2α-induced contraction (+perivascular adipose tissue), suggesting that endogenous chemerin contributes to contraction. Arteries from animals with dysfunctional endothelium (obese or hypertensive) demonstrated a pronounced contraction to chemerin-9. Finally, mesenteric arteries from obese humans demonstrate amplified contraction to chemerin-9.
Conclusions: These data support a new role for chemerin as an endogenous vasoconstrictor that operates through a receptor typically attributed to function only in immune cells.
Keywords: ChemR23; PVAT; adipose tissue; chemerin; vasoconstriction.
Figures




Similar articles
-
Role of Chemerin/ChemR23 axis as an emerging therapeutic perspective on obesity-related vascular dysfunction.J Transl Med. 2022 Mar 22;20(1):141. doi: 10.1186/s12967-021-03220-7. J Transl Med. 2022. PMID: 35317838 Free PMC article. Review.
-
The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery.Am J Physiol Heart Circ Physiol. 2016 Aug 1;311(2):H498-507. doi: 10.1152/ajpheart.00998.2015. Epub 2016 Jul 1. Am J Physiol Heart Circ Physiol. 2016. PMID: 27371688 Free PMC article.
-
Vascular chemerin from PVAT contributes to norepinephrine and serotonin-induced vasoconstriction and vascular stiffness in a sex-dependent manner.Am J Physiol Heart Circ Physiol. 2024 Dec 1;327(6):H1577-H1589. doi: 10.1152/ajpheart.00475.2024. Epub 2024 Oct 25. Am J Physiol Heart Circ Physiol. 2024. PMID: 39453435
-
Endothelin-1- and acetylcholine-mediated effects in human and rat vessels: impact of perivascular adipose tissue, diabetes, angiotensin II, and chemerin.Blood Press. 2024 Dec;33(1):2414072. doi: 10.1080/08037051.2024.2414072. Epub 2024 Oct 10. Blood Press. 2024. PMID: 39387176
-
Vascular effects of perivascular adipose tissue-derived chemerin in obesity-associated cardiovascular disease.Cardiovasc Diabetol. 2025 Jun 13;24(1):249. doi: 10.1186/s12933-025-02814-5. Cardiovasc Diabetol. 2025. PMID: 40514684 Free PMC article. Review.
Cited by
-
Novel Targets for Hypertension Drug Discovery.Curr Hypertens Rep. 2021 Mar 30;23(4):19. doi: 10.1007/s11906-021-01137-6. Curr Hypertens Rep. 2021. PMID: 33783647 Review.
-
Role of Chemerin/ChemR23 axis as an emerging therapeutic perspective on obesity-related vascular dysfunction.J Transl Med. 2022 Mar 22;20(1):141. doi: 10.1186/s12967-021-03220-7. J Transl Med. 2022. PMID: 35317838 Free PMC article. Review.
-
The Effect of Chemerin on Cardiac Parameters and Gene Expressions in Isolated Perfused Rat Heart.Balkan Med J. 2019 Jan 1;36(1):43-48. doi: 10.4274/balkanmedj.2017.1787. Epub 2018 Sep 21. Balkan Med J. 2019. PMID: 30238923 Free PMC article.
-
Chemerin Forms: Their Generation and Activity.Biomedicines. 2022 Aug 19;10(8):2018. doi: 10.3390/biomedicines10082018. Biomedicines. 2022. PMID: 36009565 Free PMC article. Review.
-
Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication.J Lipid Res. 2019 Oct;60(10):1648-1684. doi: 10.1194/jlr.R094060. Epub 2019 Jun 17. J Lipid Res. 2019. PMID: 31209153 Free PMC article. Review.
References
-
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens. 1991;13:277–296. - PubMed
-
- Chaldakov GN, Beltowsky J, Ghenev PI, Fiore M, Panayotov P, Rancic G, Aloe L. Adipoparacrinology: vascular periadventitial adipose tissue (tunica adipose) as an example. Cell Biology Int. 2011;36:327–330. - PubMed
-
- Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25:647–653. - PubMed
-
- Achike FL, To NH, Wang H, Kwan CY. Obesity, metabolic syndrome, adipocytes and vascular function: a holistic viewpoint. Clin Exp Pharmacol Physiol. 2011;38:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

