mRNA-binding protein ZFP36 is expressed in atherosclerotic lesions and reduces inflammation in aortic endothelial cells
- PMID: 23559629
- PMCID: PMC3844532
- DOI: 10.1161/ATVBAHA.113.301496
mRNA-binding protein ZFP36 is expressed in atherosclerotic lesions and reduces inflammation in aortic endothelial cells
Abstract
Objective: We studied the expression and function of an mRNA-binding protein, zinc finger protein-36 (ZFP36), in vascular endothelial cells in vivo and in vitro. We tested the hypotheses that ZFP36 regulates inflammation in vascular endothelial cells and that it functions through direct binding to target cytokine mRNAs. We also tested whether ZFP36 inhibits nuclear factor-κB-mediated transcriptional responses in vascular endothelial cells.
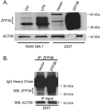
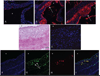
Approach and results: ZFP36 was minimally expressed in healthy aorta but was expressed in endothelial cells overlying atherosclerotic lesions in mice and humans. The protein was also expressed in macrophage foam cells of atherosclerosis. ZFP36 was expressed in human aortic endothelial cells in response to bacterial lipopolysaccharide, glucocorticoid, and forskolin, but not oxidized low-density lipoproteins or angiotensin II. Functional studies demonstrated that ZFP36 reduces the expression of inflammatory cytokines in target cells by 2 distinct mechanisms: ZFP36 inhibits nuclear factor-κB transcriptional activation and also binds to cytokine mRNAs, leading to reduced transcript stability.
Conclusions: ZFP36 is expressed in vascular endothelial cells and macrophage foam cells where it inhibits the expression of proinflammatory mRNA transcripts. The anti-inflammatory effects of ZFP36 in endothelial cells occur via both transcriptional and posttranscriptional mechanisms. Our data suggest that enhancing vascular ZFP36 expression might reduce vascular inflammation.
Keywords: AU-rich element; atherosclerosis; cytokines; endothelial cell; inflammation; zinc finger protein-36.
Conflict of interest statement
The authors report no conflicts of interest.
Figures




Similar articles
-
Post-Transcriptional Regulation of Immune Responses and Inflammatory Diseases by RNA-Binding ZFP36 Family Proteins.Front Immunol. 2021 Jul 1;12:711633. doi: 10.3389/fimmu.2021.711633. eCollection 2021. Front Immunol. 2021. PMID: 34276705 Free PMC article. Review.
-
Myeloid-specific deletion of Zfp36 protects against insulin resistance and fatty liver in diet-induced obese mice.Am J Physiol Endocrinol Metab. 2018 Oct 1;315(4):E676-E693. doi: 10.1152/ajpendo.00224.2017. Epub 2018 Mar 6. Am J Physiol Endocrinol Metab. 2018. PMID: 29509432 Free PMC article.
-
The mRNA-binding protein Zfp36 is upregulated by β-adrenergic stimulation and represses IL-6 production in 3T3-L1 adipocytes.Obesity (Silver Spring). 2012 Jan;20(1):40-7. doi: 10.1038/oby.2011.259. Epub 2011 Aug 4. Obesity (Silver Spring). 2012. PMID: 21818148 Free PMC article.
-
Intermedin inhibits macrophage foam-cell formation via tristetraprolin-mediated decay of CD36 mRNA.Cardiovasc Res. 2014 Feb 1;101(2):297-305. doi: 10.1093/cvr/cvt254. Epub 2013 Nov 18. Cardiovasc Res. 2014. PMID: 24253523
-
Postprandial lipoproteins and the molecular regulation of vascular homeostasis.Prog Lipid Res. 2013 Oct;52(4):446-64. doi: 10.1016/j.plipres.2013.06.001. Epub 2013 Jun 15. Prog Lipid Res. 2013. PMID: 23774609 Review.
Cited by
-
Exploration of ferroptosis and necroptosis-related genes and potential molecular mechanisms in psoriasis and atherosclerosis.Front Immunol. 2024 Jul 12;15:1372303. doi: 10.3389/fimmu.2024.1372303. eCollection 2024. Front Immunol. 2024. PMID: 39072329 Free PMC article.
-
RNA-binding proteins in cardiovascular biology and disease: the beat goes on.Nat Rev Cardiol. 2024 Jun;21(6):361-378. doi: 10.1038/s41569-023-00958-z. Epub 2024 Jan 2. Nat Rev Cardiol. 2024. PMID: 38163813 Review.
-
Bone marrow deficiency of mRNA decaying protein Tristetraprolin increases inflammation and mitochondrial ROS but reduces hepatic lipoprotein production in LDLR knockout mice.Redox Biol. 2020 Oct;37:101609. doi: 10.1016/j.redox.2020.101609. Epub 2020 Jun 17. Redox Biol. 2020. PMID: 32591281 Free PMC article.
-
Intercepting IRE1 kinase-FMRP signaling prevents atherosclerosis progression.EMBO Mol Med. 2022 Apr 7;14(4):e15344. doi: 10.15252/emmm.202115344. Epub 2022 Feb 22. EMBO Mol Med. 2022. PMID: 35191199 Free PMC article.
-
The control of inflammation via the phosphorylation and dephosphorylation of tristetraprolin: a tale of two phosphatases.Biochem Soc Trans. 2016 Oct 15;44(5):1321-1337. doi: 10.1042/BST20160166. Biochem Soc Trans. 2016. PMID: 27911715 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA129424/CA/NCI NIH HHS/United States
- R01 HL070531/HL/NHLBI NIH HHS/United States
- P01 HL095070/HL/NHLBI NIH HHS/United States
- K12-HD000849/HD/NICHD NIH HHS/United States
- 5P01HL095070/HL/NHLBI NIH HHS/United States
- R01 DK091841/DK/NIDDK NIH HHS/United States
- 1ZIAES090080-15/PHS HHS/United States
- R01 HL090584/HL/NHLBI NIH HHS/United States
- 5R01HD059909/HD/NICHD NIH HHS/United States
- 5R01HL070531/HL/NHLBI NIH HHS/United States
- P30 NS055077/NS/NINDS NIH HHS/United States
- R01 HD059909/HD/NICHD NIH HHS/United States
- 5R01DK091841/DK/NIDDK NIH HHS/United States
- K12 HD000849/HD/NICHD NIH HHS/United States
- R01 HD055379/HD/NICHD NIH HHS/United States
- R01-HD055379/HD/NICHD NIH HHS/United States
- R01-CA129424/CA/NCI NIH HHS/United States
- 5R01HL090584/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

