Cost-effectiveness of systemic therapies for metastatic pancreatic cancer
- PMID: 23559890
- PMCID: PMC3615875
- DOI: 10.3747/co.20.1223
Cost-effectiveness of systemic therapies for metastatic pancreatic cancer
Abstract
Purpose: Gemcitabine and capecitabine (gem-cap), gemcitabine and erlotinib (gem-e), and folfirinox (5-fluorouracil-leucovorin-irinotecan-oxaliplatin) are new treatment options for metastatic pancreatic cancer, but they are also more expensive and potentially more toxic than gemcitabine alone (gem). We conducted a cost-effectiveness analysis of these treatment options compared with gem.
Methods: A Markov model was constructed to examine costs and outcomes of gem-cap, gem-e, folfirinox, and gem in patients with metastatic pancreatic cancer from the perspective of a government health care plan. Ontario health economic and costing data (2010 Canadian dollars) were used. Efficacy data for the treatments were obtained from the published literature. Resource utilization data were derived from a chart review of consecutive metastatic patients treated for pancreatic cancer at Princess Margaret Hospital, Toronto, Ontario, 2008-2009, and supplemented with data from the literature. Utilities were obtained by surveying medical oncologists across Canada using the EQ-5D. Incremental cost-effectiveness ratios (icers) were calculated.
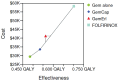
Results: The icers for gem-cap, gem-e, and folfirinox compared with gem were, respectively, CA$84,299, CA$153,631, and CA$133,184 per quality-adjusted life year (qaly). The model was driven mostly by drug acquisition costs. Given a willingness-to-pay (wtp) threshold greater than CA$130,000/qaly, folfirinox was most cost-effective treatment. When the wtp threshold was less than CA$80,000/qaly, gem alone was most cost-effective. The gem-e option was dominated by the other treatments.
Conclusions: The most cost-effective treatment for metastatic pancreatic cancer depends on the societal wtp threshold. If the societal wtp threshold were to be relatively high or if drug costs were to be substantially reduced, folfirinox might be cost-effective.
Keywords: Pancreatic cancer; chemotherapy; cost-effectiveness.
Figures

Similar articles
-
Real-World Cost-Effectiveness of First-Line Gemcitabine Plus Nab-Paclitaxel vs FOLFIRINOX in Patients With Advanced Pancreatic Cancer.JNCI Cancer Spectr. 2022 Jul 1;6(4):pkac047. doi: 10.1093/jncics/pkac047. JNCI Cancer Spectr. 2022. PMID: 35758620 Free PMC article.
-
Economic Evaluation for USA of Systemic Chemotherapies as First-Line Treatment of Metastatic Pancreatic Cancer.Pharmacoeconomics. 2018 Oct;36(10):1273-1284. doi: 10.1007/s40273-018-0678-6. Pharmacoeconomics. 2018. PMID: 29948964
-
Comparing the cost-effectiveness of FOLFIRINOX, nab-paclitaxel plus gemcitabine, gemcitabine and S-1 for the treatment of metastatic pancreatic cancer.Mol Clin Oncol. 2017 Jul;7(1):125-130. doi: 10.3892/mco.2017.1278. Epub 2017 May 30. Mol Clin Oncol. 2017. PMID: 28685089 Free PMC article.
-
Pegylated Liposomal Irinotecan Hydrochloride Trihydrate for Treating Pancreatic Cancer After Gemcitabine: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Mar;36(3):289-299. doi: 10.1007/s40273-017-0592-3. Pharmacoeconomics. 2018. PMID: 29178025 Review.
-
Comparative Safety and Efficacy of Therapeutic Options in Resectable and Advanced/Metastatic Pancreatic Cancer: A Systematic Review and Indirect Comparison.Oncol Res Treat. 2021;44(9):476-484. doi: 10.1159/000517409. Epub 2021 Jul 27. Oncol Res Treat. 2021. PMID: 34315166
Cited by
-
Cost-Effectiveness Analysis of Ofatumumab for the Treatment of Relapsing-Remitting Multiple Sclerosis in Canada.Pharmacoecon Open. 2022 Nov;6(6):859-870. doi: 10.1007/s41669-022-00363-1. Epub 2022 Sep 15. Pharmacoecon Open. 2022. PMID: 36107307 Free PMC article.
-
Role of adjuvant therapy in resected stage IA subcentimeter (T1a/T1b) pancreatic cancer.Cancer. 2019 Jan 1;125(1):57-67. doi: 10.1002/cncr.31787. Epub 2018 Nov 20. Cancer. 2019. PMID: 30457666 Free PMC article.
-
Cost-effectiveness analyses of targeted oral anti-cancer drugs: a systematic review.Pharmacoeconomics. 2014 Jul;32(7):651-80. doi: 10.1007/s40273-014-0160-z. Pharmacoeconomics. 2014. PMID: 24821281
-
Evaluation of subcutaneous rituximab administration on Canadian systemic therapy suites.Curr Oncol. 2018 Oct;25(5):300-306. doi: 10.3747/co.25.4231. Epub 2018 Oct 31. Curr Oncol. 2018. PMID: 30464679 Free PMC article.
-
Dose Escalation and Co-therapy Intensification Between Etanercept, Adalimumab, and Infliximab: The CADURA Study.Open Rheumatol J. 2017 Oct 24;11:123-135. doi: 10.2174/1874312901711010123. eCollection 2017. Open Rheumatol J. 2017. PMID: 29296125 Free PMC article.
References
-
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. - PubMed
-
- Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase iii trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–17. doi: 10.1200/JCO.2006.09.0886. - DOI - PubMed
-
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase iii trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

