Screening of multiple myeloma by polyclonal rabbit anti-human plasmacytoma cell immunoglobulin
- PMID: 23560043
- PMCID: PMC3613404
- DOI: 10.1371/journal.pone.0059117
Screening of multiple myeloma by polyclonal rabbit anti-human plasmacytoma cell immunoglobulin
Abstract
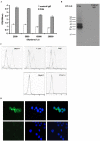
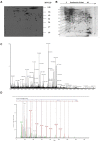
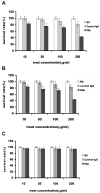
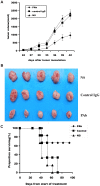
Antibody-based immunotherapy has been effectively used for tumor treatment. However, to date, only a few tumor-associated antigens (TAAs) or therapeutic targets have been identified. Identification of more immunogenic antigens is essential for improvements in multiple myeloma (MM) diagnosis and therapy. In this study, we synthesized a polyclonal antibody (PAb) by immunizing rabbits with whole human plasmacytoma ARH-77 cells and identified MM-associated antigens, including enlonase, adipophilin, and HSP90s, among others, via proteomic technologies. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay showed that 200 µg/mL PAb inhibits the proliferation of ARH-77 cells by over 50% within 48 h. Flow cytometric assay indicated that PAb treatment significantly increases the number of apoptotic cells compared with other treatments (52.1% vs. NS, 7.3% or control rabbit IgG, 9.9%). In vivo, PAb delayed tumor growth and prolonged the lifespan of mice. Terminal deoxynucleotidyl transferase dUTP nick end labeling assay showed that PAb also induces statistically significant changes in apoptosis compared with other treatments (P<0.05). We therefore conclude that PAb could be used for the effective screening and identification of TAA. PAb may have certain anti-tumor functions in vitro and in vivo. As such, its combination with proteomic technologies could be a promising approach for sieving TAA for the diagnosis and therapy of MM.
Conflict of interest statement
Figures

Similar articles
-
Polyclonal rabbit anti-murine plasmacytoma cell globulins induce myeloma cells apoptosis and inhibit tumour growth in mice.Apoptosis. 2011 Apr;16(4):370-81. doi: 10.1007/s10495-010-0568-7. Apoptosis. 2011. PMID: 21197579 Free PMC article.
-
Thymoglobulin targets multiple plasma cell antigens and has in vitro and in vivo activity in multiple myeloma.Leukemia. 2006 Oct;20(10):1863-9. doi: 10.1038/sj.leu.2404359. Epub 2006 Aug 17. Leukemia. 2006. PMID: 16932343
-
The Fc portion of UV3, an anti-CD54 monoclonal antibody, is critical for its antitumor activity in SCID mice with human multiple myeloma or lymphoma cell lines.J Immunother. 2006 Sep-Oct;29(5):489-98. doi: 10.1097/01.cji.0000210079.52554.c3. J Immunother. 2006. PMID: 16971805
-
Monoclonal antibodies to tumor antigens.Contemp Top Immunobiol. 1980;11:117-37. doi: 10.1007/978-1-4684-3701-0_3. Contemp Top Immunobiol. 1980. PMID: 6160947 Review. No abstract available.
-
The use of animal models in multiple myeloma.Morphologie. 2015 Jun;99(325):63-72. doi: 10.1016/j.morpho.2015.01.003. Epub 2015 Apr 17. Morphologie. 2015. PMID: 25898798 Review.
Cited by
-
Overcoming multiple myeloma drug resistance in the era of cancer 'omics'.Leuk Lymphoma. 2018 Mar;59(3):542-561. doi: 10.1080/10428194.2017.1337115. Epub 2017 Jun 13. Leuk Lymphoma. 2018. PMID: 28610537 Free PMC article. Review.
-
Proteomics-inspired precision medicine for treating and understanding multiple myeloma.Expert Rev Precis Med Drug Dev. 2020;5(2):67-85. doi: 10.1080/23808993.2020.1732205. Epub 2020 Feb 24. Expert Rev Precis Med Drug Dev. 2020. PMID: 34414281 Free PMC article.
References
-
- Rajkumar S, Gertz M, Kyle R, Greipp P (2002) Current therapy for multiple myeloma. Mayo Clin Proc 77: 813–822. - PubMed
-
- Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, et al. (2012) Latest advances and current challenges in the treatment of multiple myeloma. Nature Reviews Clinical Oncology 9: 135–143. - PubMed
-
- Roos R, Jansen P (2012) Novel immunotherapy strategies for multiple myeloma and other haematological malignancies. Ned Tijdschr Klin Chem Labgeneesk 37: 38–41.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

