Transforming growth factor β1 induces the expression of collagen type I by DNA methylation in cardiac fibroblasts
- PMID: 23560091
- PMCID: PMC3613378
- DOI: 10.1371/journal.pone.0060335
Transforming growth factor β1 induces the expression of collagen type I by DNA methylation in cardiac fibroblasts
Abstract
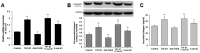
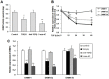
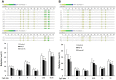
Transforming growth factor-beta (TGF-β), a key mediator of cardiac fibroblast activation, has a major influence on collagen type I production. However, the epigenetic mechanisms by which TGF-β induces collagen type I alpha 1 (COL1A1) expression are not fully understood. This study was designed to examine whether or not DNA methylation is involved in TGF-β-induced COL1A1 expression in cardiac fibroblasts. Cells isolated from neonatal Sprague-Dawley rats were cultured and stimulated with TGF-β1. The mRNA levels of COL1A1 and DNA methyltransferases (DNMTs) were determined via quantitative polymerase chain reaction and the protein levels of collagen type I were determined via Western blot as well as enzyme-linked immunosorbent assay. The quantitative methylation of the COL1A1 promoter region was analyzed using the MassARRAY platform of Sequenom. Results showed that TGF-β1 upregulated the mRNA expression of COL1A1 and induced the synthesis of cell-associated and secreted collagen type I in cardiac fibroblasts. DNMT1 and DNMT3a expressions were significantly downregulated and the global DNMT activity was inhibited when treated with 10 ng/mL of TGF-β1 for 48 h. TGF-β1 treatment resulted in a significant reduction of the DNA methylation percentage across multiple CpG sites in the rat COL1A1 promoter. Thus, TGF-β1 can induce collagen type I expression through the inhibition of DNMT1 and DNMT3a expressions as well as global DNMT activity, thereby resulting in DNA demethylation of the COL1A1 promoter. These findings suggested that the DNMT-mediated DNA methylation is an important mechanism in regulating the TGF-β1-induced COL1A1 gene expression.
Conflict of interest statement
Figures

References
-
- Brown RD, Ambler SK, Mitchell MD, Long CS (2005) The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45: 657–687. - PubMed
-
- Jugdutt BI (2003) Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Curr Drug Targets Cardiovasc Haematol Disord 3: 1–30. - PubMed
-
- Porter KE, Turner NA (2009) Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 123: 255–278. - PubMed
-
- Leask A, Abraham DJ (2004) TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

