A perfluoroaryl-cysteine S(N)Ar chemistry approach to unprotected peptide stapling
- PMID: 23560559
- PMCID: PMC3675880
- DOI: 10.1021/ja400119t
A perfluoroaryl-cysteine S(N)Ar chemistry approach to unprotected peptide stapling
Abstract
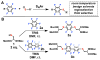
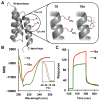
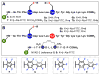
We report the discovery of a facile transformation between perfluoroaromatic molecules and a cysteine thiolate, which is arylated at room temperature. This new approach enabled us to selectively modify cysteine residues in unprotected peptides, providing access to variants containing rigid perfluoroaromatic staples. This stapling modification performed on a peptide sequence designed to bind the C-terminal domain of an HIV-1 capsid assembly polyprotein (C-CA) showed enhancement in binding, cell permeability, and proteolytic stability properties, as compared to the unstapled analog. Importantly, chemical stability of the formed staples allowed us to use this motif in the native chemical ligation-mediated synthesis of a small protein affibody that is capable of binding the human epidermal growth factor 2 receptor.
Conflict of interest statement
The authors declare no competing financial interests. Patent application covering the content of this manuscript was filed by the MIT-TLO on Sept 23, 2012.
Figures


References
-
- Hermanson GT. Bioconjugate Teachniques. Academic Press; London: 2008.
-
- Chalker JM, Bernardes GJL, Lin YA, Davis BG. Chem Asian J. 2009;4:630. - PubMed
-
- Ji X, Armstrong RN, Gilliland GL. Biochemistry. 1993;32:12949. - PubMed
-
- Chalker JM, Gunnoo SB, Boutureira O, Gerstberger SC, Fernández-Gonzáles M, Bernardes GJL, Griffin L, Hailu H, Schofield CJ, Davis BG. Chem Sci. 2011;2:1666.
-
- Langille KR, Peach ME. J Fluor Chem. 1971–72:407.
- Birchal JM, Green M, Haszeldine RN, Pitts AD. Chem Commun. 1967:338.
- Becer CR, Babiuch K, Pilz D, Hornig S, Heinze T, Gottschaldt M, Schubert US. Macromolecules. 2009;42:2387.
- Becer CR, Hoogenboom R, Schubert US. Angew Chem, Int Ed. 2009;27:4900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

