Citrus leaf blotch virus invades meristematic regions in Nicotiana benthamiana and citrus
- PMID: 23560714
- PMCID: PMC6638833
- DOI: 10.1111/mpp.12031
Citrus leaf blotch virus invades meristematic regions in Nicotiana benthamiana and citrus
Abstract
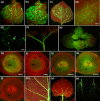
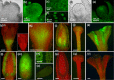
To invade systemically host plants, viruses need to replicate in the infected cells, spread to neighbouring cells through plasmodesmata and move to distal parts of the plant via sieve tubes to start new infection foci. To monitor the infection of Nicotiana benthamiana plants by Citrus leaf blotch virus (CLBV), leaves were agroinoculated with an infectious cDNA clone of the CLBV genomic RNA expressing green fluorescent protein (GFP) under the transcriptional control of a duplicate promoter of the coat protein subgenomic RNA. Fluorescent spots first appeared in agroinfiltrated leaves 11-12 days after infiltration, indicating CLBV replication. Then, after entering the phloem vascular system, CLBV was unloaded in the upper parts of the plant and invaded all tissues, including flower organs and meristems. GFP fluorescence was not visible in citrus plants infected with CLBV-GFP. Therefore, to detect CLBV in meristematic regions, Mexican lime (Citrus aurantifolia) plants were graft inoculated with CLBV, with Citrus tristeza virus (CTV), a virus readily eliminated by shoot-tip grafting in vitro, or with both simultaneously. Although CLBV was detected by hybridization and real-time reverse transcription-polymerase chain reaction (RT-PCR) in 0.2-mm shoot tips in all CLBV-inoculated plants, CTV was not detected. These results explain the difficulty in eliminating CLBV by shoot-tip grafting in vitro.
© 2013 BSPP AND JOHN WILEY & SONS LTD.
Figures


Similar articles
-
Development of a full-genome cDNA clone of Citrus leaf blotch virus and infection of citrus plants.Mol Plant Pathol. 2008 Nov;9(6):787-97. doi: 10.1111/j.1364-3703.2008.00501.x. Mol Plant Pathol. 2008. PMID: 19019007 Free PMC article.
-
Development and validation of a multiplex reverse transcription quantitative PCR (RT-qPCR) assay for the rapid detection of Citrus tristeza virus, Citrus psorosis virus, and Citrus leaf blotch virus.J Virol Methods. 2015 Aug;220:64-75. doi: 10.1016/j.jviromet.2015.04.013. Epub 2015 Apr 20. J Virol Methods. 2015. PMID: 25907469
-
Development of viral vectors based on Citrus leaf blotch virus to express foreign proteins or analyze gene function in citrus plants.Mol Plant Microbe Interact. 2012 Oct;25(10):1326-37. doi: 10.1094/MPMI-02-12-0048-R. Mol Plant Microbe Interact. 2012. PMID: 22670755
-
Under siege: virus control in plant meristems and progeny.Plant Cell. 2021 Aug 31;33(8):2523-2537. doi: 10.1093/plcell/koab140. Plant Cell. 2021. PMID: 34015140 Free PMC article. Review.
-
Citrus tristeza virus: making an ally from an enemy.Annu Rev Phytopathol. 2015;53:137-55. doi: 10.1146/annurev-phyto-080614-120012. Epub 2015 May 6. Annu Rev Phytopathol. 2015. PMID: 25973695 Review.
Cited by
-
New Plant Breeding Techniques in Citrus for the Improvement of Important Agronomic Traits. A Review.Front Plant Sci. 2020 Aug 14;11:1234. doi: 10.3389/fpls.2020.01234. eCollection 2020. Front Plant Sci. 2020. PMID: 32922420 Free PMC article. Review.
-
Autophagy Inhibits Intercellular Transport of Citrus Leaf Blotch Virus by Targeting Viral Movement Protein.Viruses. 2021 Oct 30;13(11):2189. doi: 10.3390/v13112189. Viruses. 2021. PMID: 34834995 Free PMC article.
-
Systemic Propagation of a Fluorescent Infectious Clone of a Polerovirus Following Inoculation by Agrobacteria and Aphids.Viruses. 2017 Jun 29;9(7):166. doi: 10.3390/v9070166. Viruses. 2017. PMID: 28661469 Free PMC article.
-
Genetically based location from triploid populations and gene ontology of a 3.3-mb genome region linked to Alternaria brown spot resistance in citrus reveal clusters of resistance genes.PLoS One. 2013 Oct 8;8(10):e76755. doi: 10.1371/journal.pone.0076755. eCollection 2013. PLoS One. 2013. PMID: 24116149 Free PMC article.
-
Virus-Induced Gene Silencing to Unravel the Function of Nicotiana benthamiana Genes Linked to Corolla Abscission.Methods Mol Biol. 2025;2916:139-151. doi: 10.1007/978-1-0716-4470-6_13. Methods Mol Biol. 2025. PMID: 40366593
References
-
- Adams, M.J. , Candresse, T. , Hammond, J. , Kreuze, J.F. , Martelli, G.P. , Namba, S. , Pearson, M.N. , Ryu, K.H. , Saldarelli, P. and Yoshikawa, N. (2012) Family Betaflexiviridae In: Virus Taxonomy: IXth Report of the International Committee on Taxonomy of Viruses (King A.M.Q., Adams M.J., Carstens E.B. and Lefkowitz E.J., eds), pp. 920–941. London: Elsevier Academic ; Press.
-
- Agüero, J. , Ruiz‐Ruiz, S. , Vives, M.C. , Velázquez, K. , Navarro, L. , Peña, L. , Moreno, P. and Guerri, J. (2012) Development of viral vectors based on Citrus leaf blotch virus to express foreign proteins or analyze gene function in citrus plants. Mol. Plant–Microbe. Interact. 25, 1326–1337. - PubMed
-
- Al‐Kaff, N.S. and Covey, S.N. (1996) Unusual accumulations of Cauliflower mosaic virus in local lesions, dark green leaf tissue, and roots of infected plants. Mol. Plant–Microbe. Interact. 5, 357–363.
-
- Amari, K. , Burgos, L. , Pallás, V. and Sánchez‐Pina, M.A. (2009) Vertical transmission of Prunus necrotic ringspot virus: hitch‐hiking from gametes to seedling. J. Gen. Virol. 85, 761–768. - PubMed
-
- Appiano, A. and Pennazio, S. (1972) Electron microscopy of potato meristem tips infected with Potato virus X . J. Gen. Virol. 14, 273–276. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

