Molecular clonality and antimicrobial resistance in Salmonella enterica serovars Enteritidis and Infantis from broilers in three Northern regions of Iran
- PMID: 23561048
- PMCID: PMC3623788
- DOI: 10.1186/1746-6148-9-66
Molecular clonality and antimicrobial resistance in Salmonella enterica serovars Enteritidis and Infantis from broilers in three Northern regions of Iran
Abstract
Background: Multidrug-resistant Salmonella strains are frequently encountered problems worldwide with considerable increased occurrences in recent years. The aim of this study was to investigate the occurrence and frequency of antimicrobial resistance and associated resistance genes in Salmonella isolates from broiler farms in different regions of Iran covering a time period of four years.
Results: From 2007 to 2011, 36 Salmonella strains were isolated from broiler farms located in three northern provinces of Iran. The isolates were serotyped, antimicrobial susceptibility tested, and characterized for antimicrobial resistance genes associated to the phenotype. Pulsed-field gel electrophoresis (PFGE) was applied for comparison of genetic relatedness.Two serovars were detected among the isolates; Salmonella enterica serovar Infantis (75%) and S. Enteritidis (25%). Thirty-four (94%) of the isolates exhibited resistance to nalidixic acid and ciprofloxacin caused by a single mutation in the quinolone resistance-determining region (QRDR) of gyrA. For all strains this mutation occurred in the codon of Asp87 leading to a Asp87-Tyr, Asp87-Gly or Asp87-Asn substitutions. All S. Infantis (n = 27) were resistant to tetracycline, spectinomycin, streptomycin, and sulfamethoxazole and harbored the associated resistance genes; tetA, dfrA14, aadA1, and sulI together with class 1 integrons. The isolates revealed highly similar PFGE patterns indicating clonal relatedness across different geographical locations.
Conclusion: The data provided fundamental information applicable when launching future control programs for broilers in Iran with the aim to conserve the effectiveness of important antimicrobials for treatment in humans.
Figures

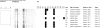
References
-
- Gast RK. In: Diseases of Poultry. 12. Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editor. Ames: Blackwell Publishing; 2008. Paratyphoid infections; pp. 636–665.
-
- Hendriksen RS, Vieira AR, Karlsmose S, Lo Fo Wong DM, Jensen AB, Wegener HC, Aarestrup FM. Global monitoring of Salmonella serovar distribution World Health Organization Foodborne Infections Network Country Data Bank; results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. 2011;8:887–900. doi: 10.1089/fpd.2010.0787. - DOI - PubMed
-
- Foley SL, Nayak R, Hanning IB, Johnson TJ, Han J, Ricke SC. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl Environ Microbiol. 2011;77:4273–4279. doi: 10.1128/AEM.00598-11. Journal of Antimicrobial Chemotherapyjac.oxfordjournals.org. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

