Artemisinin resistance in rodent malaria--mutation in the AP2 adaptor μ-chain suggests involvement of endocytosis and membrane protein trafficking
- PMID: 23561245
- PMCID: PMC3655824
- DOI: 10.1186/1475-2875-12-118
Artemisinin resistance in rodent malaria--mutation in the AP2 adaptor μ-chain suggests involvement of endocytosis and membrane protein trafficking
Abstract
Background: The control of malaria, caused by Plasmodium falciparum, is hampered by the relentless evolution of drug resistance. Because artemisinin derivatives are now used in the most effective anti-malarial therapy, resistance to artemisinin would be catastrophic. Indeed, studies suggest that artemisinin resistance has already appeared in natural infections. Understanding the mechanisms of resistance would help to prolong the effective lifetime of these drugs. Genetic markers of resistance are therefore required urgently. Previously, a mutation in a de-ubiquitinating enzyme was shown to confer artemisinin resistance in the rodent malaria parasite Plasmodium chabaudi.
Methods: Here, for a mutant P. chabaudi malaria parasite and its immediate progenitor, the in vivo artemisinin resistance phenotypes and the mutations arising using Illumina whole-genome re-sequencing were compared.
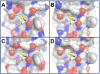
Results: An increased artemisinin resistance phenotype is accompanied by one non-synonymous substitution. The mutated gene encodes the μ-chain of the AP2 adaptor complex, a component of the endocytic machinery. Homology models indicate that the mutated residue interacts with a cargo recognition sequence. In natural infections of the human malaria parasite P. falciparum, 12 polymorphisms (nine SNPs and three indels) were identified in the orthologous gene.
Conclusion: An increased artemisinin-resistant phenotype occurs along with a mutation in a functional element of the AP2 adaptor protein complex. This suggests that endocytosis and trafficking of membrane proteins may be involved, generating new insights into possible mechanisms of resistance. The genotypes of this adaptor protein can be evaluated for its role in artemisinin responses in human infections of P. falciparum.
Figures



Similar articles
-
Experimental evolution, genetic analysis and genome re-sequencing reveal the mutation conferring artemisinin resistance in an isogenic lineage of malaria parasites.BMC Genomics. 2010 Sep 16;11:499. doi: 10.1186/1471-2164-11-499. BMC Genomics. 2010. PMID: 20846421 Free PMC article.
-
Limited polymorphisms in k13 gene in Plasmodium falciparum isolates from Dakar, Senegal in 2012-2013.Malar J. 2014 Dec 4;13:472. doi: 10.1186/1475-2875-13-472. Malar J. 2014. PMID: 25471113 Free PMC article.
-
Quantitative genome re-sequencing defines multiple mutations conferring chloroquine resistance in rodent malaria.BMC Genomics. 2012 Mar 21;13:106. doi: 10.1186/1471-2164-13-106. BMC Genomics. 2012. PMID: 22435897 Free PMC article.
-
Updates on k13 mutant alleles for artemisinin resistance in Plasmodium falciparum.J Microbiol Immunol Infect. 2018 Apr;51(2):159-165. doi: 10.1016/j.jmii.2017.06.009. Epub 2017 Jun 29. J Microbiol Immunol Infect. 2018. PMID: 28711439 Review.
-
Artemisinin susceptibility in the malaria parasite Plasmodium falciparum: propellers, adaptor proteins and the need for cellular healing.FEMS Microbiol Rev. 2021 May 5;45(3):fuaa056. doi: 10.1093/femsre/fuaa056. FEMS Microbiol Rev. 2021. PMID: 33095255 Free PMC article. Review.
Cited by
-
Mutations in Plasmodium falciparum actin-binding protein coronin confer reduced artemisinin susceptibility.Proc Natl Acad Sci U S A. 2018 Dec 11;115(50):12799-12804. doi: 10.1073/pnas.1812317115. Epub 2018 Nov 12. Proc Natl Acad Sci U S A. 2018. PMID: 30420498 Free PMC article.
-
Genetic mapping of determinants in drug resistance, virulence, disease susceptibility, and interaction of host-rodent malaria parasites.Parasitol Int. 2022 Dec;91:102637. doi: 10.1016/j.parint.2022.102637. Epub 2022 Aug 1. Parasitol Int. 2022. PMID: 35926693 Free PMC article. Review.
-
Artemisinin Action and Resistance in Plasmodium falciparum.Trends Parasitol. 2016 Sep;32(9):682-696. doi: 10.1016/j.pt.2016.05.010. Epub 2016 Jun 9. Trends Parasitol. 2016. PMID: 27289273 Free PMC article. Review.
-
Genetic screens reveal a central role for heme metabolism in artemisinin susceptibility.Nat Commun. 2020 Sep 23;11(1):4813. doi: 10.1038/s41467-020-18624-0. Nat Commun. 2020. PMID: 32968076 Free PMC article.
-
Rapid response to selection, competitive release and increased transmission potential of artesunate-selected Plasmodium chabaudi malaria parasites.PLoS Pathog. 2014 Apr 24;10(4):e1004019. doi: 10.1371/journal.ppat.1004019. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24763470 Free PMC article.
References
-
- Hartwig CL, Rosenthal AS, D'Angelo J, Griffin CE, Posner GH, Cooper RA. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem Pharmacol. 2009;77:322–336. doi: 10.1016/j.bcp.2008.10.015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

