Patch-clamp characterization of the MscS-like mechanosensitive channel from Silicibacter pomeroyi
- PMID: 23561519
- PMCID: PMC3617418
- DOI: 10.1016/j.bpj.2013.01.055
Patch-clamp characterization of the MscS-like mechanosensitive channel from Silicibacter pomeroyi
Abstract
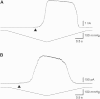
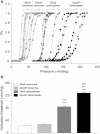
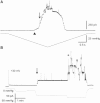
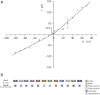
Based on sequence similarity, the sp7 gene product, MscSP, of the sulfur-compound-decomposing Gram-negative marine bacterium Silicibacter pomeroyi belongs to the family of MscS-type mechanosensitive channels. To investigate MscSP channel properties, we measured its response to membrane tension using the patch-clamp technique on either a heterologous expression system using giant spheroplasts of MJF465 Escherichia coli strain (devoid of mechanosensitive channels MscL, MscS, and MscK), or on purified MscSP protein reconstituted in azolectin liposomes. These experiments showed typical pressure-dependent gating properties of a stretch-activated channel with a current/voltage plot indicating a rectifying behavior and weak preference for anions similar to the MscS channel of E. coli. However, the MscSP channel exhibited functional differences with respect to conductance and desensitization behavior, with the most striking difference between the two channels being the lack of inactivation in MscSP compared with MscS. This seems to result from the fact that although MscSP has a Gly in an equivalent position to MscS (G113), a position that is critical for inactivation, MscSP has a Glu residue instead of an Asn in a position that was recently shown to allosterically influence MscS inactivation, N117. To our knowledge, this study describes the first electrophysiological characterization of an MscS-like channel from a marine bacterium belonging to sulfur-degrading α-proteobacteria.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
MscS inactivation: an exception rather than the rule. An extremophilic MscS reveals diversity within the family.Biophys J. 2013 Apr 2;104(7):1391-3. doi: 10.1016/j.bpj.2013.02.010. Biophys J. 2013. PMID: 23561511 Free PMC article. No abstract available.
Similar articles
-
The impact of the C-terminal domain on the gating properties of MscCG from Corynebacterium glutamicum.Biochim Biophys Acta. 2016 Jan;1858(1):130-8. doi: 10.1016/j.bbamem.2015.10.010. Epub 2015 Oct 19. Biochim Biophys Acta. 2016. PMID: 26494188
-
Characterizing the mechanosensitive response of Paraburkholderia graminis membranes.Biochim Biophys Acta Biomembr. 2020 Apr 1;1862(4):183176. doi: 10.1016/j.bbamem.2020.183176. Epub 2020 Jan 7. Biochim Biophys Acta Biomembr. 2020. PMID: 31923411
-
Patch clamp characterization of the effect of cardiolipin on MscS of E. coli.Eur Biophys J. 2015 Oct;44(7):567-76. doi: 10.1007/s00249-015-1020-2. Epub 2015 Apr 5. Eur Biophys J. 2015. PMID: 25842033
-
Mechanosensitive channels in microbes.Annu Rev Microbiol. 2010;64:313-29. doi: 10.1146/annurev.micro.112408.134106. Annu Rev Microbiol. 2010. PMID: 20825352 Review.
-
Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities.Annu Rev Physiol. 1997;59:633-57. doi: 10.1146/annurev.physiol.59.1.633. Annu Rev Physiol. 1997. PMID: 9074781 Review.
Cited by
-
MscS-like mechanosensitive channels in plants and microbes.Biochemistry. 2013 Aug 27;52(34):5708-22. doi: 10.1021/bi400804z. Epub 2013 Aug 15. Biochemistry. 2013. PMID: 23947546 Free PMC article. Review.
-
Identification and Characterization of the Neisseria gonorrhoeae MscS-Like Mechanosensitive Channel.Infect Immun. 2018 May 22;86(6):e00090-18. doi: 10.1128/IAI.00090-18. Print 2018 Jun. Infect Immun. 2018. PMID: 29581189 Free PMC article.
-
Mechanosensitive channels: feeling tension in a world under pressure.Front Plant Sci. 2014 Oct 21;5:558. doi: 10.3389/fpls.2014.00558. eCollection 2014. Front Plant Sci. 2014. PMID: 25374575 Free PMC article. Review.
-
Selectivity mechanisms in MscS-like channels: From structure to function.Channels (Austin). 2014;8(1):5-12. doi: 10.4161/chan.27107. Epub 2013 Nov 21. Channels (Austin). 2014. PMID: 24262975 Free PMC article.
-
The evolutionary 'tinkering' of MscS-like channels: generation of structural and functional diversity.Pflugers Arch. 2015 Jan;467(1):3-13. doi: 10.1007/s00424-014-1522-2. Epub 2014 May 13. Pflugers Arch. 2015. PMID: 24819593 Review.
References
-
- Martinac B., Kloda A. Evolutionary origins of mechanosensitive ion channels. Prog. Biophys. Mol. Biol. 2003;82:11–24. - PubMed
-
- Koprowski P., Kubalski A. C termini of the Escherichia coli mechanosensitive ion channel (MscS) move apart upon the channel opening. J. Biol. Chem. 2003;278:11237–11245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

