The association of polar residues in the DAP12 homodimer: TOXCAT and molecular dynamics simulation studies
- PMID: 23561520
- PMCID: PMC3617422
- DOI: 10.1016/j.bpj.2013.01.054
The association of polar residues in the DAP12 homodimer: TOXCAT and molecular dynamics simulation studies
Abstract
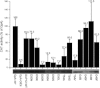
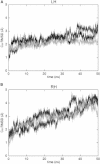
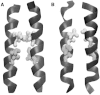
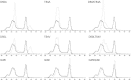
Dimerization of the transmembrane (TM) adaptor protein DAP12 plays a key role in mediating activation signals through TM-TM association with cell-surface receptors. Herein, we apply the TOXCAT assay and molecular dynamics simulation to analyze dynamics and dimerization of the TM helix of DAP12 in the membrane bilayer. In the TOXCAT assay, we performed site-specific mutagenesis of potential dimerization motifs in the DAP12 TM domain. Instead of the common GxxxG dimerization motif, mutating either of the polar residues Asp-50 and Thr-54 significantly decreased the TOXCAT signal for the dimerization of DAP12 TM domain. Furthermore, through the conformational difference between wild-type and mutant DAP12 TM homodimers, a combined coarse-grained and atomistic molecular dynamics simulation has identified both Asp-50 and Thr-54 at the dimerization interface. The experimental and computational results of the DAP12 TM dimer are in excellent agreement with the previously reported NMR structure obtained in detergent micelles. Such a combination of dynamics simulation and cell-based experiments can be applied to produce insights at the molecular level into the TM-TM association of many other transmembrane proteins.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Popot J.L., Engelman D.M. Membrane protein folding and oligomerization: the two-stage model. Biochemistry. 1990;29:4031–4037. - PubMed
-
- Melnyk R.A., Partridge A.W., Deber C.M. Transmembrane domain mediated self-assembly of major coat protein subunits from Ff bacteriophage. J. Mol. Biol. 2002;315:63–72. - PubMed
-
- Escher C., Cymer F., Schneider D. Two GxxxG-like motifs facilitate promiscuous interactions of the human ErbB transmembrane domains. J. Mol. Biol. 2009;389:10–16. - PubMed
-
- Treutlein H.R., Lemmon M.A., Brünger A.T. The glycophorin A transmembrane domain dimer: sequence-specific propensity for a right-handed supercoil of helices. Biochemistry. 1992;31:12726–12732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

