Spontaneous cardiac calcinosis in BALB/cByJ mice
- PMID: 23561935
- PMCID: PMC3567374
Spontaneous cardiac calcinosis in BALB/cByJ mice
Abstract
BALB/c mice are predisposed to dystrophic cardiac calcinosis-the mineralization of cardiac tissues, especially the right ventricular epicardium. In previous reports, the disease appeared in aged animals and had an unknown etiology. In the current study, we report a substrain of BALB/c mice (BALB/cByJ) that develops disease early and with high frequency. Here we analyzed hearts grossly to identify the presence and measure the severity of disease and to compare BALB/c substrains. Histologic analysis and fluorescent and immunofluorescent microscopy were used to characterize the calcinotic lesions. BALB/cByJ mice exhibited more frequent and severe calcium deposition than did BALB/c mice of other substrains (90% compared with 3% at 5 wk). At this age, lesions covered an average of 30% of the total ventricular surface area in BALB/cByJ mice, compared with less than 1% in other strains. In bone-marrow-chimeric mice, green fluorescent protein was used as a marker to show that the lesions contain an infiltration of cells of bone marrow origin. Lesion histology showed that calcium deposits were surrounded by fibrosis with interspersed immune cells. Lymphocytes, macrophages, and granulocytes were all present. Internalization of the gap-junction protein connexin 43 was observed in myocytes adjacent to lesions. In conclusion, BALB/cByJ mice exhibit more frequent and severe dystrophic cardiac calcinosis than do other BALB/c substrains. Our findings suggest that immune cells are actively recruited to lesions and that myocyte gap junctions are altered near lesions.
Figures



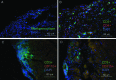
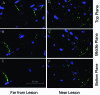
Similar articles
-
Effect of a purified diet on dystrophic cardiac calcinosis in mice.Lab Anim Sci. 1988 Aug;38(4):426-9. Lab Anim Sci. 1988. PMID: 3184851
-
High mortality with severe dystrophic cardiac calcinosis in C3H/OUJ mice fed high fat purified diets.Vet Pathol. 1988 Mar;25(2):113-8. doi: 10.1177/030098588802500202. Vet Pathol. 1988. PMID: 3363788
-
Ultrastructural changes in inherited cardiac calcinosis of DBA/2 mice.Am J Vet Res. 1987 Feb;48(2):255-61. Am J Vet Res. 1987. PMID: 3826865
-
Development of heart and aortic lesions in DBA/2NCrj mice.Lab Anim. 1986 Jan;20(1):5-8. doi: 10.1258/002367786781062151. Lab Anim. 1986. PMID: 3951193
-
New insights into myocardial arrhythmogenesis: distribution of gap-junctional coupling in normal, ischaemic and hypertrophied human hearts.Clin Sci (Lond). 1996 Jun;90(6):447-52. doi: 10.1042/cs0900447. Clin Sci (Lond). 1996. PMID: 8697713 Review.
Cited by
-
Endurance Exercise Does Not Exacerbate Cardiac Inflammation in BALB/c Mice Following mRNA COVID-19 Vaccination.Vaccines (Basel). 2024 Aug 26;12(9):966. doi: 10.3390/vaccines12090966. Vaccines (Basel). 2024. PMID: 39339998 Free PMC article.
-
Research-Relevant Conditions and Pathology of Laboratory Mice, Rats, Gerbils, Guinea Pigs, Hamsters, Naked Mole Rats, and Rabbits.ILAR J. 2021 Dec 31;62(1-2):77-132. doi: 10.1093/ilar/ilab022. ILAR J. 2021. PMID: 34979559 Free PMC article. Review.
-
C.B-17 SCID mice develop epicardial calcinosis with unaltered cardiac function.Fundam Clin Pharmacol. 2019 Feb;33(1):25-30. doi: 10.1111/fcp.12398. Epub 2018 Aug 5. Fundam Clin Pharmacol. 2019. PMID: 29959870 Free PMC article.
-
Spontaneously occurring cardiovascular lesions in commonly used laboratory animals.Cardiooncology. 2019 Jun 3;5:6. doi: 10.1186/s40959-019-0040-y. eCollection 2019. Cardiooncology. 2019. PMID: 32154013 Free PMC article. Review.
-
Characterization of murine cytomegalovirus infection and induction of calcification in Murine Aortic Vascular Smooth Muscle Cells (MOVAS).J Virol Methods. 2021 Nov;297:114270. doi: 10.1016/j.jviromet.2021.114270. Epub 2021 Aug 27. J Virol Methods. 2021. PMID: 34461152 Free PMC article.
References
-
- Aherrahrou Z, Doehring LC, Ehlers EM, Liptau H, Depping R, Linsel-Nitschke P, Kaczmarek PM, Erdmann J, Schunkert H. 2008. An alternative splice variant in Abcc6, the gene causing dystrophic calcification, leads to protein deficiency in C3H/He mice. J Biol Chem 283:7608–7615 - PubMed
-
- Ball CR, Williams WL. 1965. Spontaneous and dietary-induced cardiovascular lesions in DBA mice. Anat Rec 152:199–209 - PubMed
-
- Bellini O, Casazza AM, di Marco A. 1976. Histological and histochemical studies of myocardial lesions in BALBc/Cr mice. Lab Anim Sci 26:329–333 - PubMed
-
- Brownstein DG. 1983. Genetics of dystrophic epicardial mineralization in DBA/2 mice. Lab Anim Sci 33:247–248 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
