In vitro gene delivery with ultrasound-triggered polymer microbubbles
- PMID: 23562023
- PMCID: PMC3683598
- DOI: 10.1016/j.ultrasmedbio.2013.01.013
In vitro gene delivery with ultrasound-triggered polymer microbubbles
Abstract
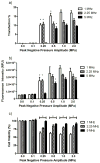
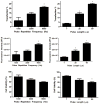
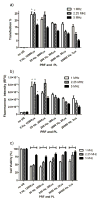
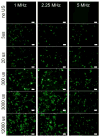
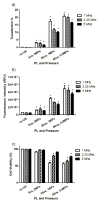
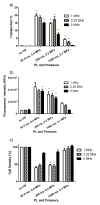
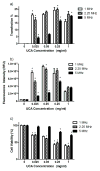
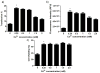
In the work described here, gene delivery using polymer microbubbles triggered by ultrasound in vitro was investigated. The effects of pressure amplitude (0-2 MPa), center frequency (1-5 MHz), pulse length (3-12,000 μs), pulse repetition frequency (5-20,000 Hz) and exposure time (0-30 s) on transfection efficiency and cell viability were examined. The effects of radiation force, calcium ion concentration and timing of treatments were also examined. Cells were successfully transfected with pressure amplitudes as low as 250 kPa. Transfection was most efficient at lower frequencies and longer pulse lengths, with a transfection efficiency of 24.2 ± 2.0% achieved using a center frequency of 1 MHz, pressure amplitude of 1 MPa, pulse length of 12,000 μs and pulse repetition frequency of 5 Hz. Gene delivery was also affected by the extracellular calcium ion concentration and the timing of treatments.
Copyright © 2013 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med Biol. 1991;17:179–85. - PubMed
-
- Azmin M, Harfield C, Ahmad Z, Edirisinghe M, Stride E. How Do Microbubbles and Ultrasound Interact? Basic Physical, Dynamic and Engineering Principles. Curr Pharm Des. 2012;18:2118–34. - PubMed
-
- Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol. 1997;23:953–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

