Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting
- PMID: 23562080
- PMCID: PMC3695242
- DOI: 10.1016/j.cmet.2013.03.004
Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting
Abstract
The delivery of blood-borne molecules conveying metabolic information to neural networks that regulate energy homeostasis is restricted by brain barriers. The fenestrated endothelium of median eminence microvessels and tight junctions between tanycytes together compose one of these. Here, we show that the decrease in blood glucose levels during fasting alters the structural organization of this blood-hypothalamus barrier, resulting in the improved access of metabolic substrates to the arcuate nucleus. These changes are mimicked by 2-deoxyglucose-induced glucoprivation and reversed by raising blood glucose levels after fasting. Furthermore, we show that VEGF-A expression in tanycytes modulates these barrier properties. The neutralization of VEGF signaling blocks fasting-induced barrier remodeling and significantly impairs the physiological response to refeeding. These results implicate glucose in the control of blood-hypothalamus exchanges through a VEGF-dependent mechanism and demonstrate a hitherto unappreciated role for tanycytes and the permeable microvessels associated with them in the adaptive metabolic response to fasting.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
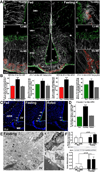
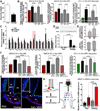

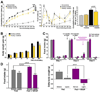
Comment in
-
How is the hungry brain like a sieve?Cell Metab. 2013 Apr 2;17(4):467-8. doi: 10.1016/j.cmet.2013.03.007. Cell Metab. 2013. PMID: 23562070 Free PMC article.
References
-
- Ambach G, Palkovits M. The blood supply of the hypothalamus of the rat. In: Morgane PJ, Panksepp J, editors. Handbook of the hypothalamus. New York: Marcel Dekker; 1979. pp. 267–377.
-
- Banks WA. Blood-brain barrier and energy balance. Obesity (Silver Spring) 2006;14(Suppl 5):234S–237S. - PubMed
-
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

