Intermediate conformations during viral fusion glycoprotein structural transition
- PMID: 23562213
- PMCID: PMC7172239
- DOI: 10.1016/j.coviro.2013.03.006
Intermediate conformations during viral fusion glycoprotein structural transition
Abstract
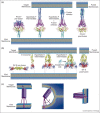
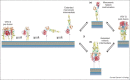
Entry of enveloped viruses into cells requires the fusion of viral and cellular membranes, driven by conformational changes in viral glycoproteins. Three different classes of viral fusion proteins have been hitherto identified based on common structural elements. Crystal structures have provided static pictures of pre-fusion and post-fusion conformations of these proteins and have revealed the dramatic reorganization of the molecules, but the transition pathway remains elusive. In this review, we will focus on recent data aiming to characterize intermediate structures during the conformational change. All these data support the existence of a pre-hairpin intermediate, but its oligomeric status is still a matter of debate.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Characterization of monomeric intermediates during VSV glycoprotein structural transition.PLoS Pathog. 2012 Feb;8(2):e1002556. doi: 10.1371/journal.ppat.1002556. Epub 2012 Feb 23. PLoS Pathog. 2012. PMID: 22383886 Free PMC article.
-
Viral membrane fusion.Virology. 2015 May;479-480:498-507. doi: 10.1016/j.virol.2015.03.043. Epub 2015 Apr 10. Virology. 2015. PMID: 25866377 Free PMC article. Review.
-
Dynamic Viral Glycoprotein Machines: Approaches for Probing Transient States That Drive Membrane Fusion.Viruses. 2016 Jan 11;8(1):15. doi: 10.3390/v8010015. Viruses. 2016. PMID: 26761026 Free PMC article. Review.
-
Structural basis of viral invasion: lessons from paramyxovirus F.Curr Opin Struct Biol. 2007 Aug;17(4):427-36. doi: 10.1016/j.sbi.2007.08.016. Epub 2007 Sep 17. Curr Opin Struct Biol. 2007. PMID: 17870467 Free PMC article. Review.
-
The structural basis of paramyxovirus invasion.Trends Microbiol. 2006 Jun;14(6):243-6. doi: 10.1016/j.tim.2006.04.004. Epub 2006 May 4. Trends Microbiol. 2006. PMID: 16678421 Free PMC article. Review.
Cited by
-
Hepatitis C Virus Envelope Glycoprotein E1 Forms Trimers at the Surface of the Virion.J Virol. 2015 Oct;89(20):10333-46. doi: 10.1128/JVI.00991-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246575 Free PMC article.
-
Probing the paramyxovirus fusion (F) protein-refolding event from pre- to postfusion by oxidative footprinting.Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):E2596-605. doi: 10.1073/pnas.1408983111. Epub 2014 Jun 9. Proc Natl Acad Sci U S A. 2014. PMID: 24927585 Free PMC article.
-
Modulation of the pH Stability of Influenza Virus Hemagglutinin: A Host Cell Adaptation Strategy.Biophys J. 2016 Jun 7;110(11):2293-2301. doi: 10.1016/j.bpj.2016.04.035. Biophys J. 2016. PMID: 27276248 Free PMC article. Review.
-
The three lives of viral fusion peptides.Chem Phys Lipids. 2014 Jul;181:40-55. doi: 10.1016/j.chemphyslip.2014.03.003. Epub 2014 Apr 2. Chem Phys Lipids. 2014. PMID: 24704587 Free PMC article. Review.
-
Interference with the production of infectious viral particles and bimodal inhibition of replication are broadly conserved antiviral properties of IFITMs.PLoS Pathog. 2017 Sep 28;13(9):e1006610. doi: 10.1371/journal.ppat.1006610. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28957419 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

