Glycophorin A transmembrane domain dimerization in plasma membrane vesicles derived from CHO, HEK 293T, and A431 cells
- PMID: 23562404
- PMCID: PMC3679245
- DOI: 10.1016/j.bbamem.2013.03.022
Glycophorin A transmembrane domain dimerization in plasma membrane vesicles derived from CHO, HEK 293T, and A431 cells
Abstract
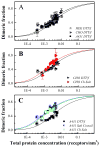
Membrane protein interactions, which underlie biological function, take place in the complex cellular membrane environment. Plasma membrane derived vesicles are a model system which allows the interactions between membrane proteins to be studied without the need for their extraction, purification, and reconstitution into lipid bilayers. Plasma membrane vesicles can be produced from different cell lines and by different methods, providing a rich variety of native-like model systems. With these choices, however, questions arise as to how the different types of vesicle preparations affect the interactions between membrane proteins. Here we address this question using the glycophorin A transmembrane domain (GpA) as a model system. We compare the dimerization of GpA in six different vesicle preparations derived from Chinese hamster ovary (CHO), Human Embryonic Kidney 293T (HEK 293T) and A431 cells. We accomplish this with the use of a FRET-based method which yields the FRET efficiency, the donor concentration, and the acceptor concentration in each vesicle. We show that the vesicle preparation protocol has no statistically significant effect on GpA dimerization. Based on these results, we propose that any of the six plasma membrane preparations investigated here can be used as a model system for studies of membrane protein interactions.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Measuring the energetics of membrane protein dimerization in mammalian membranes.J Am Chem Soc. 2010 Mar 17;132(10):3628-35. doi: 10.1021/ja910692u. J Am Chem Soc. 2010. PMID: 20158179 Free PMC article.
-
Glycophorin A dimerization and band 3 interaction during erythroid membrane biogenesis: in vivo studies in human glycophorin A transgenic mice.Blood. 2001 May 1;97(9):2872-8. doi: 10.1182/blood.v97.9.2872. Blood. 2001. PMID: 11313283
-
Lipid-modulated sequence-specific association of glycophorin A in membranes.Biophys J. 2010 Jul 7;99(1):284-92. doi: 10.1016/j.bpj.2010.04.005. Biophys J. 2010. PMID: 20655857 Free PMC article.
-
Membrane microheterogeneity: Förster resonance energy transfer characterization of lateral membrane domains.Eur Biophys J. 2010 Mar;39(4):589-607. doi: 10.1007/s00249-009-0547-5. Epub 2009 Oct 21. Eur Biophys J. 2010. PMID: 19844701 Review.
-
Lipid domains in supported lipid bilayer for atomic force microscopy.Methods Mol Biol. 2007;400:503-13. doi: 10.1007/978-1-59745-519-0_34. Methods Mol Biol. 2007. PMID: 17951756 Review.
Cited by
-
Strong dimerization of wild-type ErbB2/Neu transmembrane domain and the oncogenic Val664Glu mutant in mammalian plasma membranes.Biochim Biophys Acta. 2014 Sep;1838(9):2326-30. doi: 10.1016/j.bbamem.2014.03.001. Epub 2014 Mar 11. Biochim Biophys Acta. 2014. PMID: 24631664 Free PMC article.
-
A Rigorous Framework for Calculating Protein-Protein Binding Affinities in Membranes.J Chem Theory Comput. 2023 Dec 26;19(24):9077-9092. doi: 10.1021/acs.jctc.3c00941. Epub 2023 Dec 13. J Chem Theory Comput. 2023. PMID: 38091976 Free PMC article.
-
Mechanism of FGF receptor dimerization and activation.Nat Commun. 2016 Jan 4;7:10262. doi: 10.1038/ncomms10262. Nat Commun. 2016. PMID: 26725515 Free PMC article.
-
Addressing the Excessive Aggregation of Membrane Proteins in the MARTINI Model.J Chem Theory Comput. 2021 Apr 13;17(4):2513-2521. doi: 10.1021/acs.jctc.0c01253. Epub 2021 Mar 15. J Chem Theory Comput. 2021. PMID: 33720709 Free PMC article.
-
FGFR3 transmembrane domain interactions persist in the presence of its extracellular domain.Biophys J. 2013 Jul 2;105(1):165-71. doi: 10.1016/j.bpj.2013.05.053. Biophys J. 2013. PMID: 23823235 Free PMC article.
References
-
- White SH, Ladokhin AS, Jayasinghe S, Hristova K. How membranes shape protein structure. J Biol Chem. 2001;276:32395–32398. - PubMed
-
- White SH, Wimley WC. Membrane protein folding and stability: Physical principles. Annu Rev Biophys Biomol Struc. 1999;28:319–365. - PubMed
-
- Popot JL, Engelman DM. Membrane protein folding and oligomerization: The 2-stage model. Biochemistry. 1990;29:4031–4037. - PubMed
-
- Cymer F, Veerappan A, Schneider D. Transmembrane helix-helix interactions are modulated by the sequence context and by lipid bilayer properties. Biochimica et Biophysica Acta-Biomembranes. 2012;1818:963–973. - PubMed
-
- Fink A, Sal-Man N, Gerber D, Shai Y. Transmembrane domains interactions within the membrane milieu: Principles, advances and challenges. Biochimica et Biophysica Acta-Biomembranes. 2012;1818:974–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

