Results of a follow-up study to the randomized Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT)
- PMID: 23562431
- PMCID: PMC3823756
- DOI: 10.1016/j.jalz.2012.11.012
Results of a follow-up study to the randomized Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT)
Abstract
Objectives: The Alzheimer's Disease Anti-inflammatory Prevention Trial Follow-up Study (ADAPT-FS) was designed to evaluate the efficacy of naproxen and celecoxib for the primary prevention of Alzheimer's disease (AD) several years after cessation of treatment in ADAPT.
Methods: ADAPT was a randomized, double-masked, multicenter clinical trial of naproxen or celecoxib vs placebo (1:1:1.5 assignment ratio) at six U.S.-based clinics. The trial enrolled 2528 people between 2001 and 2004. Treatments were discontinued in December 2004 and participants were monitored regularly until 2007. In 2010 and 2011, ADAPT-FS screened 1537 participants by telephone and, if indicated, examined them in person using standardized clinical assessments. The primary outcome was time to diagnosis of AD. Death index searches were performed for participants not located.
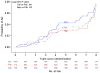
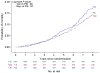
Results: Eighty-nine additional AD events were identified (24 celecoxib, 25 naproxen, and 40 placebo) yielding a total of 161 events (48 [6.6% of randomized participants] celecoxib, 43 [6.0%] naproxen, and 70 [6.5%] placebo) across ADAPT and ADAPT-FS. Adjusted hazard ratios (HRs) comparing each treatment with placebo showed no overall reduction in risk of AD: HR celecoxib vs placebo, 1.03 (95% confidence interval [CI], 0.72-1.50; P = .86); HR naproxen vs placebo, 0.92 (95% CI, 0.62-1.35; P = .66). There were 349 deaths (110 [15.2%] celecoxib, 96 [13.4%] naproxen, and 143 [13.2%] placebo). Risk of death was similar for the naproxen- and placebo-assigned groups (HR, 0.99; 95% CI, 0.76-1.28; P = .93) and slightly higher for celecoxib compared with the placebo-assigned group (HR, 1.15; 95% CI, 0.90-1.48; P = .27).
Conclusions: These results acquired during a follow-up of approximately 7 years (which included a median of less than 1.5 years of treatment) do not support the hypothesis that celecoxib or naproxen prevent AD in adults with a family history of dementia.
Keywords: Alzheimer's disease; Celecoxib; Clinical trial; Naproxen; Nonsteroidal anti-inflammatory drug; Prevention.
Copyright © 2013 The Alzheimer's Association. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Szekely CA, Town T, Zandi PP. NSAIDs for the chemoprevention of Alzheimer's disease. Subcell Biochem. 2007;42:229–48. - PubMed
-
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–5. - PubMed
-
- Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–4. - PubMed
-
- McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

