Protein kinase D2 regulates migration and invasion of U87MG glioblastoma cells in vitro
- PMID: 23562655
- PMCID: PMC3715702
- DOI: 10.1016/j.yexcr.2013.03.029
Protein kinase D2 regulates migration and invasion of U87MG glioblastoma cells in vitro
Abstract
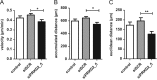
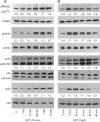
Glioblastoma multiforme (GBM) is the most common malignant brain tumor, which, despite combined modality treatment, reoccurs and is invariably fatal for affected patients. Recently, a member of the serine/threonine protein kinase D (PRKD) family, PRKD2, was shown to be a potent mediator of glioblastoma growth. Here we studied the role of PRKD2 in U87MG glioblastoma cell migration and invasion in response to sphingosine-1-phosphate (S1P), an activator of PRKD2 and a GBM mitogen. Time-lapse microscopy demonstrated that random cell migration was significantly diminished in response to PRKD2 silencing. The pharmacological PRKD family inhibitor CRT0066101 decreased chemotactic migration and invasion across uncoated or matrigel-coated Transwell inserts. Silencing of PRKD2 attenuated migration and invasion of U87MG cells even more effectively. In terms of downstream signaling, CRT0066101 prevented PRKD2 autophosphorylation and inhibited p44/42 MAPK and to a smaller extent p54/46 JNK and p38 MAPK activation. PRKD2 silencing impaired activation of p44/42 MAPK and p54/46 JNK, downregulated nuclear c-Jun protein levels and decreased c-Jun(S73) phosphorylation without affecting the NFκB pathway. Finally, qPCR array analyses revealed that silencing of PRKD2 downregulates mRNA levels of integrin alpha-2 and -4 (ITGA2 and -4), plasminogen activator urokinase (PLAU), plasminogen activator urokinase receptor (PLAUR), and matrix metallopeptidase 1 (MMP1). Findings of the present study identify PRKD2 as a potential target to interfere with glioblastoma cell migration and invasion, two major determinants contributing to recurrence of glioblastoma after multimodality treatment.
Keywords: C-Jun; Glioblastoma; Invasion; MAPK; PRKD2; Sphingosine-1-phosphate.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., Curschmann J., Janzer R.C., Ludwin S.K., Gorlia T., Allgeier A., Lacombe D., Cairncross J.G., Eisenhauer E., Mirimanoff R.O. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. - PubMed
-
- Parsons D.W., Jones S., Zhang X., Lin J.C., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.M., Gallia G.L., Olivi A., McLendon R., Rasheed B.A., Keir S., Nikolskaya T., Nikolsky Y., Busam D.A., Tekleab H., Diaz L.A., Jr., Hartigan J., Smith D.R., Strausberg R.L., Marie S.K., Shinjo S.M., Yan H., Riggins G.J., Bigner D.D., Karchin R., Papadopoulos N., Parmigiani G., Vogelstein B., Velculescu V.E., Kinzler K.W. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. - PMC - PubMed
-
- Friedl P., Locker J., Sahai E., Segall J.E. Classifying collective cancer cell invasion. Nat. Cell Biol. 2012;14:777–783. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

