Retinal deimination and PAD2 levels in retinas from donors with age-related macular degeneration (AMD)
- PMID: 23562679
- PMCID: PMC3683981
- DOI: 10.1016/j.exer.2013.03.017
Retinal deimination and PAD2 levels in retinas from donors with age-related macular degeneration (AMD)
Abstract
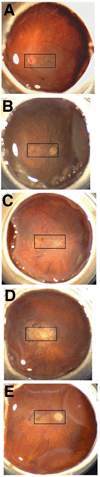
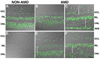
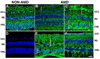
Deimination is a form of protein posttranslational modification carried out by the peptidyl arginine deiminases (PADs) enzymes. PAD2 is the principal deiminase expressed in the retina. Elevated levels of PAD2 and protein deimination are present in a number of human neurological diseases, with or without ocular manifestation. To define the association of deimination with the pathogenesis of age-related macular degeneration (AMD), we studied protein deimination and PAD2 levels in retinas of AMD donor eyes compared to age-matched non-AMD retinas. Eyes from non-AMD and AMD donors were fixed in 4% paraformaldehyde and 0.5% glutaraldehyde in phosphate buffer. Retina and retinal pigment epithelium (RPE) from donor eyes were processed for immunohistochemical detection and western blotting using antibodies to PAD2 and citrulline residues. The ganglion cell, inner plexiform, inner nuclear and outer nuclear layers were labeled by both PAD2 and citrulline antibodies. Changes in the localization of deiminated residues and PAD2 were evident as the retinal layers were remodeled coincident with photoreceptor degeneration in AMD retinas. Immunodetection of either PAD2 or citrulline residues could not be evaluated in the RPE layer due to the high autofluorescence levels in this layer. Interestingly, higher deimination immunoreactivity was detected in AMD retinal lysates. However, no significant changes in PAD2 were detected in the AMD and non-AMD retinas and RPE lysates. Our observations show increased levels of protein deimination but not PAD2 in AMD retinas and RPE, suggesting a reduced rate of turnover of deiminated proteins in these AMD retinas.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

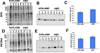
References
-
- Acharya NK, Nagele EP, Han M, Coretti NJ, DeMarshall C, Kosciuk MC, Boulos PA, Nagele RG. Neuronal PAD4 expression and protein citrullination: possible role in production of autoantibodies associated with neurodegenerative disease. J. Autoimmun. 2012;38:369–380. - PubMed
-
- Asaga H, Ishigami A. Protein deimination in the rat brain after kainate administration: citrulline-containing proteins as a novel marker of neurodegeneration. Neurosci. Lett. 2001;299:5–8. - PubMed
-
- Asaga H, Akiyama K, Ohsawa T, Ishigami A. Increased and type II-specific expression of peptidylarginine deiminase in activated microglia but not hyperplastic astrocytes following kainic acid-evoked neurodegeneration in the rat brain. Neurosci. Lett. 2002;326:129–132. - PubMed
-
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. - PubMed
-
- Bando H, Shadrach KG, Rayborn ME, Crabb JW, Hollyfield JG. Clathrin and adaptin accumulation in drusen, Bruch's membrane and choroid in AMD and non-AMD donor eyes. Exp. Eye Res. 2007;84:135–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

