Characterization of genome-reduced fission yeast strains
- PMID: 23563150
- PMCID: PMC3664816
- DOI: 10.1093/nar/gkt233
Characterization of genome-reduced fission yeast strains
Abstract
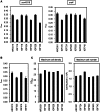
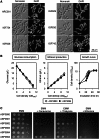
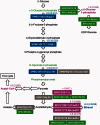
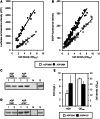
The Schizosaccharomyces pombe genome is one of the smallest among the free-living eukaryotes. We further reduced the S. pombe gene number by large-scale gene deletion to identify a minimal gene set required for growth under laboratory conditions. The genome-reduced strain has four deletion regions: 168.4 kb in the left arm of chromosome I, 155.4 kb in the right arm of chromosome I, 211.7 kb in the left arm of chromosome II and 121.6 kb in the right arm of chromosome II. The deletions corresponded to a loss of 223 genes of the original ~5100. The quadruple-deletion strain, with a total deletion size of 657.3 kb, showed a decreased ability to uptake glucose and some amino acids in comparison with the parental strain. The strain also showed increased gene expression of the mating pheromone M-factor precursor and the nicotinamide adenine dinucleotide phosphate -specific glutamate dehydrogenase. There was also a 2.7-fold increase in the concentration of cellular adenosine triphosphate, and levels of the heterologous proteins, enhanced green fluorescent protein and secreted human growth hormone were increased by 1.7- and 1.8-fold, respectively. The transcriptome data from this study have been submitted to the Gene Expression Omnibus (GEO: http://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE38620 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=vjkxjewuywgcovc&acc=GSE38620).
Figures



Similar articles
-
Schizosaccharomyces pombe minimum genome factory.Biotechnol Appl Biochem. 2007 Mar;46(Pt 3):147-55. doi: 10.1042/BA20060106. Biotechnol Appl Biochem. 2007. PMID: 17300221 Review.
-
Data for chromosome contacts and matched transcription profiles at three cell cycle phases in the fission yeast.Genom Data. 2015 Jan 20;4:12-6. doi: 10.1016/j.gdata.2015.01.005. eCollection 2015 Jun. Genom Data. 2015. PMID: 26484169 Free PMC article.
-
Bulk Segregant Analysis Reveals the Genetic Basis of a Natural Trait Variation in Fission Yeast.Genome Biol Evol. 2015 Nov 27;7(12):3496-510. doi: 10.1093/gbe/evv238. Genome Biol Evol. 2015. PMID: 26615217 Free PMC article.
-
Genes involved in glucose repression and oxidative stress response in the fission yeast Schizosaccharomyces pombe.Genet Mol Res. 2011 Nov 8;10(4):4041-7. doi: 10.4238/2011.November.8.4. Genet Mol Res. 2011. PMID: 22095476
-
Production of heterologous proteins using the fission-yeast (Schizosaccharomyces pombe) expression system.Biotechnol Appl Biochem. 2009 Jun 22;53(Pt 4):227-35. doi: 10.1042/BA20090048. Biotechnol Appl Biochem. 2009. PMID: 19531030 Review.
Cited by
-
Deletion of a hybrid NRPS-T1PKS biosynthetic gene cluster via Latour gene knockout system in Saccharopolyspora pogona and its effect on butenyl-spinosyn biosynthesis and growth development.Microb Biotechnol. 2021 Nov;14(6):2369-2384. doi: 10.1111/1751-7915.13694. Epub 2020 Oct 31. Microb Biotechnol. 2021. PMID: 33128503 Free PMC article.
-
Systematic analysis reveals the prevalence and principles of bypassable gene essentiality.Nat Commun. 2019 Mar 1;10(1):1002. doi: 10.1038/s41467-019-08928-1. Nat Commun. 2019. PMID: 30824696 Free PMC article.
-
Hsp90 Maintains Proteostasis of the Galactose Utilization Pathway To Prevent Cell Lethality.Mol Cell Biol. 2016 Apr 15;36(9):1412-24. doi: 10.1128/MCB.01064-15. Print 2016 May. Mol Cell Biol. 2016. PMID: 26951197 Free PMC article.
-
Top-Down, Knowledge-Based Genetic Reduction of Yeast Central Carbon Metabolism.mBio. 2022 Oct 26;13(5):e0297021. doi: 10.1128/mbio.02970-21. Epub 2022 Sep 21. mBio. 2022. PMID: 36129294 Free PMC article.
-
Promising non-model microbial cell factories obtained by genome reduction.Front Bioeng Biotechnol. 2024 Aug 5;12:1427248. doi: 10.3389/fbioe.2024.1427248. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39161352 Free PMC article. Review.
References
-
- Medema MH, van Raaphorst R, Takano E, Breitling R. Computational tools for the synthetic design of biochemical pathways. Nat. Rev. Microbiol. 2012;10:191–202. - PubMed
-
- Fujio T. Minimum genome factory: innovation in bioprocesses through genome science. Biotechnol. Appl. Biochem. 2007;46:145–146. - PubMed
-
- Posfai G, Plunkett G, Feher T, Frisch D, Keil GM, Umenhoffer K, Kolisnychenko V, Stahl B, Sharma SS, de Arruda M, et al. Emergent properties of reduced-genome Escherichia coli. Science. 2006;312:1044–1046. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

