Ribosomal protein S7 regulates arsenite-induced GADD45α expression by attenuating MDM2-mediated GADD45α ubiquitination and degradation
- PMID: 23563151
- PMCID: PMC3664810
- DOI: 10.1093/nar/gkt223
Ribosomal protein S7 regulates arsenite-induced GADD45α expression by attenuating MDM2-mediated GADD45α ubiquitination and degradation
Abstract
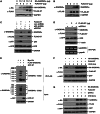
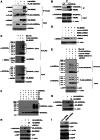
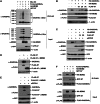
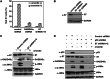
The stress-responding protein, GADD45α, plays important roles in cell cycle checkpoint, DNA repair and apoptosis. In our recent study, we demonstrate that GADD45α undergoes a dynamic ubiquitination and degradation in vivo, which process can be blocked by the cytotoxic reagent, arsenite, resulting in GADD45α accumulation to activate JNKs cell death pathway, thereby revealing a novel mechanism for the cellular GADD45α functional regulation. But the factors involved in GADD45α stability modulations are unidentified. Here, we demonstrated that MDM2 was an E3 ubiquitin ligase for GADD45α. One of MDM2-binding partner, ribosomal protein S7, interacted with and stabilized GADD45α through preventing the ubiquitination and degradation of GADD45α mediated by MDM2. This novel function of S7 is unrelated to p53 but seems to depend on S7/MDM2 interaction, for the S7 mutant lacking MDM2-binding ability lost its function to stabilize GADD45α. Further investigations indicated that arsenite treatment enhanced S7-MDM2 interaction, resulting in attenuation of MDM2-dependent GADD45α ubiquitination and degradation, thereby leading to GADD45α-dependent cell death pathway activation. Silencing S7 expression suppressed GADD45α-dependent cytotoxicity induced by arsenite. Our findings thus identify a novel function of S7 in control of GADD45α stabilization under both basal and stress conditions and its significance in mediating arsenite-induced cellular stress.
Figures



References
-
- Gao M, NIng G, Chuanshu H, Lun S. Diverse roles of GADD45a in stres signaling. Curr. Protein Pep. Sci. 2009;10:388–394. - PubMed
-
- Barreto G, Schafer A, Marhold J, Stach D, Swaminathan SK, Handa V, Doderlein G, Maltry N, Wu W, Lyko F, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

