Intra-amniotic LPS amplifies hyperoxia-induced airway hyperreactivity in neonatal rats
- PMID: 23563192
- PMCID: PMC3707085
- DOI: 10.1038/pr.2013.58
Intra-amniotic LPS amplifies hyperoxia-induced airway hyperreactivity in neonatal rats
Retraction in
-
Retraction Note: Intra-amniotic LPS amplifies hyperoxia-induced airway hyperreactivity in neonatal rats.Pediatr Res. 2019 Nov;86(5):677. doi: 10.1038/s41390-019-0529-y. Pediatr Res. 2019. PMID: 31481720 Free PMC article.
Abstract
Background: We previously showed that intra-amniotic lipopolysaccharide (LPS) amplifies alveolar hypoplasia induced by postnatal hyperoxia. We determined whether the priming effect of intra-amniotic LPS amplifies hyperoxia-induced airway hyperreactivity (AHR).
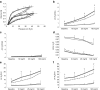
Methods: LPS or normal saline was injected into the amniotic cavities of pregnant rats at the 20th day of gestation. After birth, rat pups were exposed to 60% O₂ or air for 14 d. On postnatal day 14, rat pups underwent forced oscillometry, which included a challenge with nebulized methacholine, and the lungs were harvested for morphological studies.
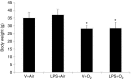
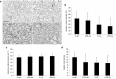
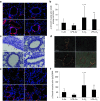
Results: Hyperoxia significantly increased airway reactivity and decreased compliance. Intra-amniotic LPS further increased hyperoxia-induced AHR but did not further impair respiratory system compliance. Hyperoxia-induced changes in lung parenchymal and small airway morphology were not further altered by intra-amniotic LPS. However, combined exposure to intra-amniotic LPS and hyperoxia increased the proportion of degranulating mast cells in the hilar airways.
Conclusion: Intra-amniotic LPS amplified postnatal hyperoxia-induced AHR. This was associated with increased airway mast cell degranulation, which has previously been linked with hyperoxia-induced AHR. There were no morphologic changes of parenchyma or airways that would account for the LPS augmentation of hyperoxia-induced AHR.
Figures
Comment in
-
Findings of Research Misconduct.Fed Regist. 2019 Nov 7;84(216):60097-60098. Fed Regist. 2019. PMID: 37547121 Free PMC article. No abstract available.
References
-
- Van Marter LJ, Dammann O, Allred EN, Developmental Epidemiology Network Investigators et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–6. - PubMed
-
- Choi CW, Kim BI, Hong JS, Kim EK, Kim HS, Choi JH. Bronchopulmonary dysplasia in a rat model induced by intra-amniotic inflammation and postnatal hyperoxia: morphometric aspects. Pediatr Res. 2009;65:323–7. - PubMed
-
- Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–7. - PubMed
-
- Tomashefski JF, Jr, Oppermann HC, Vawter GF, Reid LM. Bronchopulmonary dysplasia: a morphometric study with emphasis on the pulmonary vasculature. Pediatr Pathol. 1984;2:469–87. - PubMed
-
- Robin B, Kim YJ, Huth J, et al. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37:236–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

