Mechanism of interleukin-1α transcriptional regulation of S100A9 in a human epidermal keratinocyte cell line
- PMID: 23563247
- PMCID: PMC3719387
- DOI: 10.1016/j.bbagrm.2013.03.010
Mechanism of interleukin-1α transcriptional regulation of S100A9 in a human epidermal keratinocyte cell line
Abstract
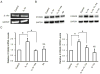
S100A9 is a calcium-binding protein and subunit of antimicrobial calprotectin complex (S100A8/A9). Produced by neutrophils, monocytes/macrophages and keratinocytes, S100A9 expression increases in response to inflammation. For example, IL-1α produced by epithelial cells acts autonomously on the same cells to induce the expression of S100A8/A9 and cellular differentiation. Whereas it is well known that IL-1α and members of the IL-10 family of cytokines upregulate S100A8 and S100A9 in several cell lineages, the pathway and mechanism of IL-1α-dependent transcriptional control of S100A9 in epithelial cells are not established. Modeled using human epidermal keratinocytes (HaCaT cells), IL-1α stimulated the phosphorylation of p38 MAPK and induced S100A9 expression, which was blocked by IL-1 receptor antagonist, RNAi suppression of p38, or a p38 MAPK inhibitor. Transcription of S100A9 in HaCaT cells depended on nucleotides -94 to -53 in the upstream promoter region, based upon the use of deletion constructs and luciferase reporter activity. Within the responsive promoter region, IL-1α increased the binding activity of CCAAT/enhancer binding protein β (C/EBPβ). Mutated C/EBPβ binding sequences or C/EBPβ-specific siRNA inhibited the S100A9 transcriptional response. Hence, IL-1α is strongly suggested to increase S100A9 expression in a human epidermal keratinocyte cell line by signaling through the IL-1 receptor and p38 MAPK, increasing C/EBPβ-dependent transcriptional activity.
Keywords: C/EBPβ; ERK; IL-1 receptor; IL-1R1; IL-1α; JNK; Keratinocytes; S100A9; TLR5; Toll-like receptor 5; c-JUN N-terminal kinase; extracellular-regulated kinase; p38; p38 kinase; siRNA; small interfering RNA.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Modulation of calprotectin in human keratinocytes by keratinocyte growth factor and interleukin-1alpha.Immunol Cell Biol. 2010 Mar-Apr;88(3):328-33. doi: 10.1038/icb.2009.104. Epub 2010 Jan 12. Immunol Cell Biol. 2010. PMID: 20065999
-
The antimicrobial heterodimer S100A8/S100A9 (calprotectin) is upregulated by bacterial flagellin in human epidermal keratinocytes.J Invest Dermatol. 2010 Oct;130(10):2423-30. doi: 10.1038/jid.2010.158. Epub 2010 Jun 17. J Invest Dermatol. 2010. PMID: 20555353
-
Regulation of calprotectin expression by interleukin-1alpha and transforming growth factor-beta in human gingival keratinocytes.J Periodontal Res. 2007 Feb;42(1):1-7. doi: 10.1111/j.1600-0765.2005.00857.x. J Periodontal Res. 2007. PMID: 17214633
-
IL-1 receptor regulates S100A8/A9-dependent keratinocyte resistance to bacterial invasion.Mucosal Immunol. 2012 Jan;5(1):66-75. doi: 10.1038/mi.2011.48. Epub 2011 Oct 26. Mucosal Immunol. 2012. PMID: 22031183 Free PMC article.
-
Expression of S100A8 is induced by interleukin‑1α in TR146 epithelial cells through a mechanism involving CCAAT/enhancer binding protein β.Mol Med Rep. 2019 Mar;19(3):2413-2420. doi: 10.3892/mmr.2019.9864. Epub 2019 Jan 15. Mol Med Rep. 2019. PMID: 30664211
Cited by
-
Autonomous immunity in mucosal epithelial cells: fortifying the barrier against infection.Microbes Infect. 2016 Jun;18(6):387-398. doi: 10.1016/j.micinf.2016.03.008. Epub 2016 Mar 19. Microbes Infect. 2016. PMID: 27005450 Free PMC article. Review.
-
S100-A9 protein in exosomes derived from follicular fluid promotes inflammation via activation of NF-κB pathway in polycystic ovary syndrome.J Cell Mol Med. 2020 Jan;24(1):114-125. doi: 10.1111/jcmm.14642. Epub 2019 Sep 30. J Cell Mol Med. 2020. PMID: 31568644 Free PMC article.
-
S100A8/A9 stimulates keratinocyte proliferation in the development of squamous cell carcinoma of the skin via the receptor for advanced glycation-end products.PLoS One. 2015 Mar 26;10(3):e0120971. doi: 10.1371/journal.pone.0120971. eCollection 2015. PLoS One. 2015. PMID: 25811984 Free PMC article.
-
Antimicrobial peptides: Defending the mucosal epithelial barrier.Front Oral Health. 2022 Aug 1;3:958480. doi: 10.3389/froh.2022.958480. eCollection 2022. Front Oral Health. 2022. PMID: 35979535 Free PMC article. Review.
-
Survival prediction in patients with head and neck squamous cell carcinoma and novel mechanistic insights of S100A8/A9.Discov Oncol. 2024 Nov 15;15(1):657. doi: 10.1007/s12672-024-01540-w. Discov Oncol. 2024. PMID: 39546127 Free PMC article.
References
-
- Volz A, Korge BP, Compton JG, Ziegler A, Steinert PM, Mischke D. Physical mapping of a functional cluster of epidermal differentiation genes on chromosome 1q21. Genomics. 1993;18:92–99. - PubMed
-
- Fagerhol MK, Andersson KB, Naess-Andresen CF, Brandtzaeg P, Dale I. Calprotectin (The L1 leukocyte protein) In: Smith VL, Dedman JR, editors. Stimulus response coupling The role of intracellular calcium-binding proteins. CRC Press; Boca Raton, Ann Arbor, Boston, MA: 1990. pp. 187–210.
-
- Eversole LR, Miyasaki KT, Christensen RE. The distribution of the antimicrobial protein, calprotectin, in normal oral keratinocytes. Arch Oral Biol. 1992;37:963–968. - PubMed
-
- Kerkhoff C, Klempt M, Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9) Biochim Biophys Acta. 1998;1448:200–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

