Endothelial SRF/MRTF ablation causes vascular disease phenotypes in murine retinae
- PMID: 23563308
- PMCID: PMC3635718
- DOI: 10.1172/JCI64201
Endothelial SRF/MRTF ablation causes vascular disease phenotypes in murine retinae
Abstract
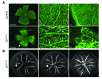
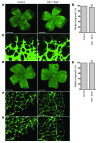
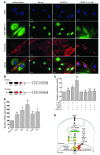
Retinal vessel homeostasis ensures normal ocular functions. Consequently, retinal hypovascularization and neovascularization, causing a lack and an excess of vessels, respectively, are hallmarks of human retinal pathology. We provide evidence that EC-specific genetic ablation of either the transcription factor SRF or its cofactors MRTF-A and MRTF-B, but not the SRF cofactors ELK1 or ELK4, cause retinal hypovascularization in the postnatal mouse eye. Inducible, EC-specific deficiency of SRF or MRTF-A/MRTF-B during postnatal angiogenesis impaired endothelial tip cell filopodia protrusion, resulting in incomplete formation of the retinal primary vascular plexus, absence of the deep plexi, and persistence of hyaloid vessels. All of these features are typical of human hypovascularization-related vitreoretinopathies, such as familial exudative vitreoretinopathies including Norrie disease. In contrast, conditional EC deletion of Srf in adult murine vessels elicited intraretinal neovascularization that was reminiscent of the age-related human pathologies retinal angiomatous proliferation and macular telangiectasia. These results indicate that angiogenic homeostasis is ensured by differential stage-specific functions of SRF target gene products in the developing versus the mature retinal vasculature and suggest that the actin-directed MRTF-SRF signaling axis could serve as a therapeutic target in the treatment of human vascular retinal diseases.
Figures







Similar articles
-
Elk3 deficiency causes transient impairment in post-natal retinal vascular development and formation of tortuous arteries in adult murine retinae.PLoS One. 2014 Sep 9;9(9):e107048. doi: 10.1371/journal.pone.0107048. eCollection 2014. PLoS One. 2014. PMID: 25203538 Free PMC article.
-
Endothelial depletion of murine SRF/MRTF provokes intracerebral hemorrhagic stroke.Proc Natl Acad Sci U S A. 2015 Aug 11;112(32):9914-9. doi: 10.1073/pnas.1509047112. Epub 2015 Jul 28. Proc Natl Acad Sci U S A. 2015. PMID: 26221020 Free PMC article.
-
BRG1 (Brahma-Related Gene 1) Promotes Endothelial Mrtf Transcription to Establish Embryonic Capillary Integrity.Arterioscler Thromb Vasc Biol. 2017 Sep;37(9):1674-1682. doi: 10.1161/ATVBAHA.117.309785. Epub 2017 Jul 20. Arterioscler Thromb Vasc Biol. 2017. PMID: 28729363 Free PMC article.
-
SRF/MRTF-A and liver cirrhosis: Pathologic associations.J Dig Dis. 2022 Nov;23(11):614-619. doi: 10.1111/1751-2980.13150. Epub 2023 Feb 7. J Dig Dis. 2022. PMID: 36601855 Review.
-
Development and pathology of the hyaloid, choroidal and retinal vasculature.Int J Dev Biol. 2004;48(8-9):1045-58. doi: 10.1387/ijdb.041895ms. Int J Dev Biol. 2004. PMID: 15558494 Review.
Cited by
-
Mechanical regulation of chromatin and transcription.Nat Rev Genet. 2022 Oct;23(10):624-643. doi: 10.1038/s41576-022-00493-6. Epub 2022 May 23. Nat Rev Genet. 2022. PMID: 35606569 Review.
-
The lysophosphatidic acid-regulated signal transduction network in ovarian cancer cells and its role in actomyosin dynamics, cell migration and entosis.Theranostics. 2023 Mar 21;13(6):1921-1948. doi: 10.7150/thno.81656. eCollection 2023. Theranostics. 2023. PMID: 37064875 Free PMC article.
-
YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation.J Clin Invest. 2017 Sep 1;127(9):3441-3461. doi: 10.1172/JCI93825. Epub 2017 Aug 14. J Clin Invest. 2017. PMID: 28805663 Free PMC article.
-
mDia2 is an important mediator of MRTF-A-dependent regulation of breast cancer cell migration.Mol Biol Cell. 2024 Oct 1;35(10):ar133. doi: 10.1091/mbc.E24-01-0008. Epub 2024 Aug 28. Mol Biol Cell. 2024. PMID: 39196658 Free PMC article.
-
G Protein-Coupled Receptor and RhoA-Stimulated Transcriptional Responses: Links to Inflammation, Differentiation, and Cell Proliferation.Mol Pharmacol. 2015 Jul;88(1):171-80. doi: 10.1124/mol.115.097857. Epub 2015 Apr 22. Mol Pharmacol. 2015. PMID: 25904553 Free PMC article. Review.
References
-
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

