Small RNAs derived from the 5' end of tRNA can inhibit protein translation in human cells
- PMID: 23563448
- PMCID: PMC3710361
- DOI: 10.4161/rna.24285
Small RNAs derived from the 5' end of tRNA can inhibit protein translation in human cells
Abstract
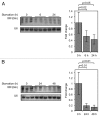
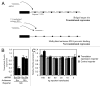
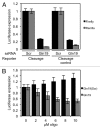
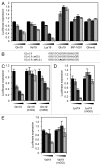
Recently, it has been shown that tRNA molecules can be processed into small RNAs that are derived from both the 5' and 3' termini. To date, the function of these tRNA fragments (tRFs) derived from the 5' end of tRNA has not been investigated in depth. We present evidence that conserved residues in tRNA, present in all 5' tRFs, can inhibit the process of protein translation without the need for complementary target sites in the mRNA. These results implicate 5' tRFs in a new mechanism of gene regulation by small RNAs in human cells.
Keywords: short tRNA fragment; small RNA; tRF; tRNA; translation repression.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
