Comparative transcriptomics of infectious spores from the fungal pathogen Histoplasma capsulatum reveals a core set of transcripts that specify infectious and pathogenic states
- PMID: 23563482
- PMCID: PMC3675998
- DOI: 10.1128/EC.00069-13
Comparative transcriptomics of infectious spores from the fungal pathogen Histoplasma capsulatum reveals a core set of transcripts that specify infectious and pathogenic states
Abstract
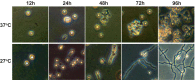
Histoplasma capsulatum is a fungal pathogen that infects both healthy and immunocompromised hosts. In regions where it is endemic, H. capsulatum grows in the soil and causes respiratory and systemic disease when inhaled by humans. An interesting aspect of H. capsulatum biology is that it adopts specialized developmental programs in response to its environment. In the soil, it grows as filamentous chains of cells (mycelia) that produce asexual spores (conidia). When the soil is disrupted, conidia aerosolize and are inhaled by mammalian hosts. Inside a host, conidia germinate into yeast-form cells that colonize immune cells and cause disease. Despite the ability of conidia to initiate infection and disease, they have not been explored on a molecular level. We developed methods to purify H. capsulatum conidia, and we show here that these cells germinate into filaments at room temperature and into yeast-form cells at 37°C. Conidia internalized by macrophages germinate into the yeast form and proliferate within macrophages, ultimately lysing the host cells. Similarly, infection of mice with purified conidia is sufficient to establish infection and yield viable yeast-form cells in vivo. To characterize conidia on a molecular level, we performed whole-genome expression profiling of conidia, yeast, and mycelia from two highly divergent H. capsulatum strains. In parallel, we used homology and protein domain analysis to manually annotate the predicted genes of both strains. Analyses of the resultant data defined sets of transcripts that reflect the unique molecular states of H. capsulatum conidia, yeast, and mycelia.
Figures


References
-
- McNeil MM, Nash SL, Hajjeh RA, Phelan MA, Conn LA, Plikaytis BD, Warnock DW. 2001. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin. Infect. Dis. 33:641–647 - PubMed
-
- Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. 2006. Hospitalizations for endemic mycoses: a population-based national study. Clin. Infect. Dis. 42:822–825 - PubMed
-
- Helmbright AL, Larsh HW. 1956. The size of the spores of Histoplasma capsulatum. Public Health Monogr. 39:81–83 - PubMed
-
- Kasuga T, White TJ, Koenig G, McEwen J, Restrepo A, Castañeda E, Da Silva Lacaz C, Heins-Vaccari EM, De Freitas RS, Zancopé-Oliveira RM, Qin Z, Negroni R, Carter DA, Mikami Y, Tamura M, Taylor ML, Miller GF, Poonwan N, Taylor JW. 2003. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol. Ecol. 12:3383–3401 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

