A comparative analysis of the epidemiological impact and disease cost-savings of HPV vaccines in France
- PMID: 23563511
- PMCID: PMC3903902
- DOI: 10.4161/hv.22994
A comparative analysis of the epidemiological impact and disease cost-savings of HPV vaccines in France
Abstract
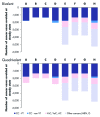
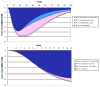
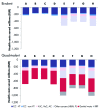
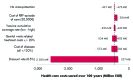
The aim was to compare the epidemiological and economic impact of 16/18 bivalent and 6/11/16/18 quadrivalent HPV vaccination in France, considering differences in licensed outcomes, protection against non-vaccine HPV types and prevention of HPV-6/11-related diseases. The differential impact of the two vaccines was evaluated using a published model adapted to the French setting. The target population was females aged 14-23 y and the time horizon was 100 y. A total of eight different scenarios compared vaccination impact in terms of reduction in HPV-16/18-associated carcinomas (cervical, vulvar, vaginal, anal, penile and head and neck), HPV-6/11-related genital warts and recurrent respiratory papillomatosis, and incremental reduction in cervical cancer due to potential cross-protection. Quadrivalent vaccine was associated with total discounted cost savings ranging from EUR 544-1,020 million vs. EUR 177-538 million with the bivalent vaccination (100-y time horizon). Genital wart prevention thanks to quadrivalent HPV vaccination accounted for EUR 306-380 million savings (37-56% of costs saved). In contrast, the maximal assumed cross-protection against cervical cancer resulted in EUR 13-33 million savings (4%). Prevention of vulvar, vaginal and anal cancers accounted for additional EUR 71-89 million savings (13%). In France, the quadrivalent HPV vaccination would result in significant incremental epidemiological and economic benefits vs. the bivalent vaccination, driven primarily by prevention of genital. The present analysis is the first in the French setting to consider the impact of HPV vaccination on all HPV diseases and non-vaccine types.
Keywords: France; HPV vaccine; cost-consequence; human papillomavirus.
Figures
Similar articles
-
Public health impact and cost-effectiveness of switching from bivalent to nonavalent vaccine for human papillomavirus in Norway: incorporating the full health impact of all HPV-related diseases.J Med Econ. 2023 Jan-Dec;26(1):1085-1098. doi: 10.1080/13696998.2023.2250194. J Med Econ. 2023. PMID: 37608730
-
A cost-effectiveness analysis of adult human papillomavirus vaccination strategies in Italy.Hum Vaccin Immunother. 2025 Dec;21(1):2474891. doi: 10.1080/21645515.2025.2474891. Epub 2025 Mar 17. Hum Vaccin Immunother. 2025. PMID: 40094352 Free PMC article.
-
Cost-effectiveness of HPV vaccination in the context of high cervical cancer incidence and low screening coverage.Vaccine. 2017 Nov 1;35(46):6329-6335. doi: 10.1016/j.vaccine.2017.08.083. Epub 2017 Sep 9. Vaccine. 2017. PMID: 28899625
-
Quadrivalent human papillomavirus vaccine.Clin Infect Dis. 2007 Sep 1;45(5):609-7. doi: 10.1086/520654. Epub 2007 Jul 25. Clin Infect Dis. 2007. PMID: 17682997 Review.
-
Quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine (Gardasil®): a review of its use in the prevention of premalignant genital lesions, genital cancer and genital warts in women.Drugs. 2010 Dec 24;70(18):2449-74. doi: 10.2165/11204920-000000000-00000. Drugs. 2010. PMID: 21142263 Review.
Cited by
-
Comparative Cost-Effectiveness Analysis of Two Different Two-Dose Human Papillomavirus Vaccines in Malaysia.Asian Pac J Cancer Prev. 2018 Apr 25;19(4):933-940. doi: 10.22034/APJCP.2018.19.4.933. Asian Pac J Cancer Prev. 2018. PMID: 29693347 Free PMC article.
-
"A systematic literature review of the epidemiology, clinical, economic and humanistic burden in recurrent respiratory papillomatosis".Respir Res. 2024 Dec 18;25(1):430. doi: 10.1186/s12931-024-03057-w. Respir Res. 2024. PMID: 39696284 Free PMC article.
-
Communicating Benefits from Vaccines Beyond Preventing Infectious Diseases.Infect Dis Ther. 2020 Sep;9(3):467-480. doi: 10.1007/s40121-020-00312-7. Epub 2020 Jun 24. Infect Dis Ther. 2020. PMID: 32583334 Free PMC article. Review.
-
Developing Michigan Cancer Foundation 7 Cells with Stable Expression of E7 Gene of Human Papillomavirus Type 16.Iran J Pathol. 2016 Winter;11(1):41-6. Iran J Pathol. 2016. PMID: 26870142 Free PMC article.
References
-
- Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–14. doi: 10.1086/597071. - DOI - PubMed
-
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006;24 Suppl 3:S3/35–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
