Rapid and multi-level characterization of trastuzumab using sheathless capillary electrophoresis-tandem mass spectrometry
- PMID: 23563524
- PMCID: PMC4169039
- DOI: 10.4161/mabs.23995
Rapid and multi-level characterization of trastuzumab using sheathless capillary electrophoresis-tandem mass spectrometry
Abstract
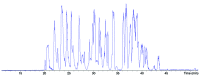
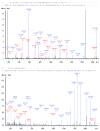
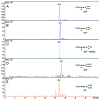
Monoclonal antibodies (mAbs) are highly complex proteins that display a wide range of microheterogeneity that requires multiple analytical methods for full structure assessment and quality control. As a consequence, the characterization of mAbs on different levels is particularly product - and time - consuming. This work presents the characterization of trastuzumab sequence using sheathless capillary electrophoresis (referred as CESI) - tandem mass spectrometry (CESI-MS/MS). Using this bottom-up proteomic-like approach, CESI-MS/MS provided 100% sequence coverage for both heavy and light chain via peptide fragment fingerprinting (PFF) identification. The result was accomplished in a single shot, corresponding to the analysis of 100 fmoles of digest. The same analysis also enabled precise characterization of the post-translational hot spots of trastuzumab, used as a representative widely marketed therapeutic mAb, including the structural confirmation of the five major N-glycoforms.
Keywords: CESI-MS/MS; N-linked glycosylation; mass spectrometry; microheterogeneity; microvariant; monoclonal antibody; peptide mapping; posttranslational modifications; sheathless capillary electrophoresis.
Figures




Similar articles
-
Monoclonal antibodies biosimilarity assessment using transient isotachophoresis capillary zone electrophoresis-tandem mass spectrometry.MAbs. 2014;6(6):1464-73. doi: 10.4161/mabs.36305. MAbs. 2014. PMID: 25484058 Free PMC article.
-
Full antibody primary structure and microvariant characterization in a single injection using transient isotachophoresis and sheathless capillary electrophoresis-tandem mass spectrometry.Anal Chem. 2014 Sep 16;86(18):9074-81. doi: 10.1021/ac502378e. Epub 2014 Sep 3. Anal Chem. 2014. PMID: 25141158
-
Complementary middle-down and intact monoclonal antibody proteoform characterization by capillary zone electrophoresis - mass spectrometry.Electrophoresis. 2018 Aug;39(16):2069-2082. doi: 10.1002/elps.201800067. Epub 2018 Jun 5. Electrophoresis. 2018. PMID: 29749064 Free PMC article.
-
[Recent progress in capillary electrophoresis-based high-sensitivity proteomics].Se Pu. 2020 Oct 8;38(10):1125-1132. doi: 10.3724/SP.J.1123.2020.03003. Se Pu. 2020. PMID: 34213109 Review. Chinese.
-
[Capillary electrophoresis-mass spectrometry and its application to proteomic analysis].Se Pu. 2020 Oct 8;38(10):1117-1124. doi: 10.3724/SP.J.1123.2020.03005. Se Pu. 2020. PMID: 34213108 Review. Chinese.
Cited by
-
Optimal Synthetic Glycosylation of a Therapeutic Antibody.Angew Chem Weinheim Bergstr Ger. 2016 Feb 12;128(7):2407-2413. doi: 10.1002/ange.201508723. Epub 2016 Jan 12. Angew Chem Weinheim Bergstr Ger. 2016. PMID: 27546920 Free PMC article.
-
Recent trends of capillary electrophoresis-mass spectrometry in proteomics research.Mass Spectrom Rev. 2019 Nov;38(6):445-460. doi: 10.1002/mas.21599. Epub 2019 Aug 12. Mass Spectrom Rev. 2019. PMID: 31407381 Free PMC article. Review.
-
Detailed mass analysis of structural heterogeneity in monoclonal antibodies using native mass spectrometry.Nat Protoc. 2014 Apr;9(4):967-76. doi: 10.1038/nprot.2014.057. Epub 2014 Mar 27. Nat Protoc. 2014. PMID: 24675736
-
Characterization of N-Linked Glycosylation in a Monoclonal Antibody Produced in NS0 Cells Using Capillary Electrophoresis with Laser-Induced Fluorescence Detection.Pharmaceuticals (Basel). 2013 Mar 21;6(3):393-406. doi: 10.3390/ph6030393. Pharmaceuticals (Basel). 2013. PMID: 24276024 Free PMC article.
-
9th annual European Antibody Congress, November 11-13, 2013, Geneva, Switzerland.MAbs. 2014 Mar-Apr;6(2):309-26. doi: 10.4161/mabs.27903. Epub 2014 Jan 17. MAbs. 2014. PMID: 24492298 Free PMC article.
References
-
- Damen CWN, Chen W, Chakraborty AB, van Oosterhout M, Mazzeo JR, Gebler JC, et al. Electrospray ionization quadrupole ion-mobility time-of-flight mass spectrometry as a tool to distinguish the lot-to-lot heterogeneity in N-glycosylation profile of the therapeutic monoclonal antibody trastuzumab. J Am Soc Mass Spectrom. 2009;20:2021–33. doi: 10.1016/j.jasms.2009.07.017. - DOI - PubMed
-
- Committee for medicinal products for human use (CHMP), European Medicines Agency (EMA). Guideline on development, production, characterization and specifications for monoclonal antibodies and related products 2009; EMEA/CHMP/BWP/157653/2007.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
