Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF)
- PMID: 23563576
- PMCID: PMC3746839
- DOI: 10.1093/eurjhf/hft052
Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF)
Abstract
Aims: Although the focus of therapeutic intervention has been on neurohormonal pathways thought to be harmful in heart failure (HF), such as the renin-angiotensin-aldosterone system (RAAS), potentially beneficial counter-regulatory systems are also active in HF. These promote vasodilatation and natriuresis, inhibit abnormal growth, suppress the RAAS and sympathetic nervous system, and augment parasympathetic activity. The best understood of these mediators are the natriuretic peptides which are metabolized by the enzyme neprilysin. LCZ696 belongs to a new class of drugs, the angiotensin receptor neprilysin inhibitors (ARNIs), which both block the RAAS and augment natriuretic peptides.
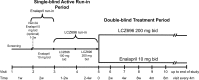
Methods: Patients with chronic HF, NYHA class II-IV symptoms, an elevated plasma BNP or NT-proBNP level, and an LVEF of ≤40% were enrolled in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortailty and morbidity in Heart Failure trial (PARADIGM-HF). Patients entered a single-blind enalapril run-in period (titrated to 10 mg b.i.d.), followed by an LCZ696 run-in period (100 mg titrated to 200 mg b.i.d.). A total of 8436 patients tolerating both periods were randomized 1:1 to either enalapril 10 mg b.i.d. or LCZ696 200 mg b.i.d. The primary outcome is the composite of cardiovascular death or HF hospitalization, although the trial is powered to detect a 15% relative risk reduction in cardiovascular death.
Perspectives: PARADIGM-HF will determine the place of the ARNI LCZ696 as an alternative to enalapril in patients with systolic HF. PARADIGM-HF may change our approach to neurohormonal modulation in HF.
Trial registration: NCT01035255.
Keywords: ACE inhibitor; Angiotensin receptor blocker; Angiotensin receptor neprilysin inhibitor; Chronic heart failure; LCZ696; Natriuretic peptides; Neprilysin; Neutral endopeptidase; Renin–angiotensin.
References
-
- Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–254. - PubMed
-
- Swedberg K. Importance of neuroendocrine activation in chronic heart failure. Impact on treatment strategies. Eur J Heart Fail. 2000;2:229–233. - PubMed
-
- Packer M. Beta-blockade in the management of chronic heart failure. Another step in the conceptual evolution of a neurohormonal model of the disease. Eur Heart J. 1996;17:21–23. (Suppl B) - PubMed
-
- McMurray JJ. CONSENSUS to EMPHASIS: the overwhelming evidence which makes blockade of the renin–angiotensin–aldosterone system the cornerstone of therapy for systolic heart failure. Eur J Heart Fail. 2011;13:929–936. - PubMed
-
- Shibata MC, Flather MD, Wang D. Systematic review of the impact of beta blockers on mortality and hospital admissions in heart failure. Eur J Heart Fail. 2001;3:351–357. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous